Lobaplatin-based regimens outperform cisplatin for metastatic breast cancer after anthracyclines and taxanes treatment
- PMID: 30108440
- PMCID: PMC6087814
- DOI: 10.1016/j.sjbs.2018.01.011
Lobaplatin-based regimens outperform cisplatin for metastatic breast cancer after anthracyclines and taxanes treatment
Abstract
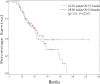
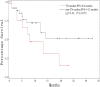
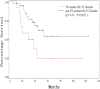
The goal of this study was to assess the antitumor efficacy and safety of lobaplatin-based regimens as the second line of treatment in patients with metastatic breast cancer (MBC) resistant to anthracyclines and taxanes, compared with that of cisplatin-based regimens. During August 2012 to April 2015, 87 patients who received lobaplatin-based regimens or cisplatin-based regimens were included. Medical records of the patients noted that lobaplatin (30 mg/m2) or cisplatin (25 mg/m2), combined with another chemotherapeutic agent such as Gemcitabine (1000 mg/m2) or Vinorelbine (25 mg/m2), was intravenously given to the patients on a basis of twenty-one days as one treatment cycle. All the patients were followed until August 2017. The endpoint of this study was progression-free survival (PFS), overall survival (OS), and estimated objective response rate (RR). Safety and drug tolerability data were also obtained. Lobaplatin-based regimens prolonged PFS compared to cisplatin-based regimens (median 13.2 vs 4.7 months, hazard ratio = 0.37, 95% confidence intervals: 0.21-0.67, P = .0007), while OS was not significantly different between the two groups (hazard ratio = 0.72, 95% confidence intervals: 0.40-1.30, P = .2767), as was objective RR (37.8% vs 33.4%, = 0.19, P = .6653). Nausea/vomiting and renal injury were more frequent with cisplatin-based regimens. Our results show that lobaplatin-based regimens are superior to cisplatin in terms of efficacy and are better tolerated.
Keywords: Breast cancer; Cisplatin; Eastern Cooperative Oncology Group, ECOG; Lobaplatin; Metastatic; National Cancer Institute Common Toxicity Criteria for Adverse Events, NCI-CTCAE; Resistant; Response Evaluation Criteria in Solid Tumors, RECIST; cisplatin and gemcitabine, GP; cisplatin and vinorelbine, NP; complete response, CR; confidence interval, CI; estrogen receptor, ER; granulocyte-colony stimulating factor, G-CSF; hazard ratio, HR; human epidermal growth factor receptor 2, HER-2; lobaplatin and gemcitabine, GL; lobaplatin and vinorelbine, NL; lymph nodes, LN; metastatic breast cancer, MBC; non-small-cell lung cancer, NSCLC; overall survival, OS; partial response, PR; performance scale, PS; platinum-based compounds, PBCs; progesterone receptor, PR; progression-free survival, PFS; progressive disease, PD; response rate, RR; stable disease, SD; standard error, SE; time to progression, TTP; triple negative breast cancer, TNBC.
Figures
References
-
- Achanta G. Interaction of p53 and DNA-PK in response to nucleoside analogues: potential role as a sensor complex for DNA damage. Cancer Res. 2001;61:8723–8729. - PubMed
-
- Aogi K. Efficacy and safety of ixabepilone in taxane-resistant patients with metastatic breast cancer previously treated with anthracyclines: results of a phase II study in Japan. Cancer Chemother. Pharmacol. 2013;71:1427–1433. - PubMed
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous