Inhibition of Diacylglycerol Lipase Impairs Fear Extinction in Mice
- PMID: 30108473
- PMCID: PMC6080414
- DOI: 10.3389/fnins.2018.00479
Inhibition of Diacylglycerol Lipase Impairs Fear Extinction in Mice
Abstract
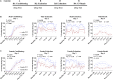
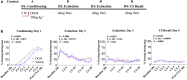
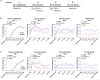
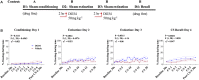
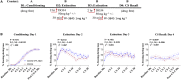
Elucidating the underlying molecular mechanisms regulating fear and extinction learning may offer insights that can lead to novel treatments for debilitating anxiety and trauma-related disorders including posttraumatic stress disorder. The endocannabinoid (eCB) system is a retrograde inhibitory signaling pathway involved in regulating central responses to stress. The eCB 2-arachidonoylglycerol (2-AG) has recently been proposed to serve as a homeostatic signal mitigating adverse effects of stress exposure, however, less well understood is 2-AG's role in fear learning and fear extinction. In this study, we have sought to explore 2-AG's role in fear conditioning and fear extinction by disrupting 2-AG synthesis utilizing the DAGL inhibitor (DO34) and DAGLα knock-out mice (DAGLα-/-). We found that DAGLα-/- mice, and male and female C57B6/J mice treated with DO34, exhibited impairment in extinction learning in an auditory cue fear-conditioning paradigm. DO34 did not increase unconditioned freezing. Interestingly, inhibition of fatty-acid amide hydrolase was not able to restore normal fear extinction in DO34-treated mice suggesting increased Anandamide cannot compensate for deficient 2-AG signaling in the regulation of fear extinction. Moreover, augmentation of CB1R signaling with tetrahydrocannabinol also failed to restore normal fear extinction in DO34-treated mice. Overall, these data support the hypothesis that DAGLα plays an important role in fear extinction learning. Although genetic and pharmacological disruption of DAGL activity causes widespread lipidomic remodeling, these data combined with previous studies putatively suggest that deficient 2-AG signaling could be a susceptibility endophenotype for the development of trauma-related psychiatric disorders.
Keywords: 2-arachidonoylglycerol; FAAH; cannabinoid; endocannabinoid; extinction; fear; stress.
Figures
References
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

