An Oral Combination of Vitamins A, C, E, and Mg++ Improves Auditory Thresholds in Age-Related Hearing Loss
- PMID: 30108480
- PMCID: PMC6079267
- DOI: 10.3389/fnins.2018.00527
An Oral Combination of Vitamins A, C, E, and Mg++ Improves Auditory Thresholds in Age-Related Hearing Loss
Abstract
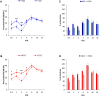
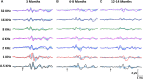
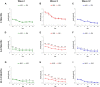
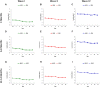
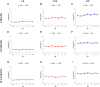
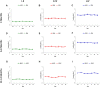
The increasing rate of age-related hearing loss (ARHL), with its subsequent reduction in quality of life and increase in health care costs, requires new therapeutic strategies to reduce and delay its impact. The goal of this study was to determine if ARHL could be reduced in a rat model by administering a combination of antioxidant vitamins A, C, and E acting as free radical scavengers along with Mg++, a known powerful cochlear vasodilator (ACEMg). Toward this goal, young adult, 3 month-old Wistar rats were divided into two groups: one was fed with a diet composed of regular chow ("normal diet," ND); the other received a diet based on chow enriched in ACEMg ("enhanced diet," ED). The ED feeding began 10 days before the noise stimulation. Auditory brainstem recordings (ABR) were performed at 0.5, 1, 2, 4, 8, 16, and 32 kHz at 3, 6-8, and 12-14 months of age. No differences were observed at 3 months of age, in both ND and ED animals. At 6-8 and 12-14 months of age there were significant increases in auditory thresholds and a reduction in the wave amplitudes at all frequencies tested, compatible with progressive development of ARHL. However, at 6-8 months threshold shifts in ED rats were significantly lower in low and medium frequencies, and wave amplitudes were significantly larger at all frequencies when compared to ND rats. In the oldest animals, differences in the threshold shift persisted, as well as in the amplitude of the wave II, suggesting a protective effect of ACEMg on auditory function during aging. These findings indicate that oral ACEMg may provide an effective adjuvant therapeutic intervention for the treatment of ARHL, delaying the progression of hearing impairment associated with age.
Keywords: age-related hearing loss; aging; auditory brainstem response; hearing loss; magnesium; micronutrients; presbycusis; vitamins.
Figures




References
-
- Alvarado J. C., Fuentes-Santamaría V., Gabaldón-Ull M. C., Jareño-Flores T., Miller J. M., Juiz J. M. (2016). Noise-induced “Toughening” effect in wistar rats: enhanced auditory brainstem responses are related to calretinin and nitric oxide synthase upregulation. Front. Neuroanat. 10:19 10.3389/fnana.2016.00019 - DOI - PMC - PubMed
-
- Alvarado J. C., Fuentes-Santamaría V., Melgar-Rojas P., Gabaldón-Ull M. C., Juiz J. M. (2015a). Short-Duration and Long-Term Noise Overstimulation Could Increase Hearing Loss in Aging Wistar Rat. SENC Communications Abstracts Book. Available at: https://www.congresomovil.com/senc2015
-
- Alvarado J. C., Fuentes-Santamaría V., Melgar-Rojas P., Valero M. L., Gabaldón-Ull M. C., Miller J. M., et al. (2015b). Synergistic effects of free radical scavengers and cochlear vasodilators: a new otoprotective strategy for age-related hearing loss. Front. Aging Neurosci. 7:86. 10.3389/fnagi.2015.00086 - DOI - PMC - PubMed
LinkOut - more resources
Full Text Sources
Other Literature Sources

