PolyQ Tract Toxicity in SCA1 is Length Dependent in the Absence of CAG Repeat Interruption
- PMID: 30108484
- PMCID: PMC6080413
- DOI: 10.3389/fncel.2018.00200
PolyQ Tract Toxicity in SCA1 is Length Dependent in the Absence of CAG Repeat Interruption
Abstract
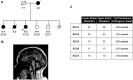
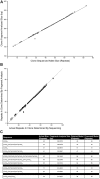
Spinocerebellar ataxia type 1 (SCA1) is an autosomal dominant neurodegenerative disorder caused by an expansion of a polyglutamine tract within the ATXN1 gene. Normal alleles have been reported to range from 6 to 35 repeats, intermediate alleles from 36 to 38 repeats and fully penetrant pathogenic alleles have at least 39 repeats. This distribution was based on relatively few samples and the narrow intermediate range makes the accuracy of the repeat sizing crucial for interpreting and reporting diagnostic tests, which can vary between laboratories. Here, we examine the distribution of 6378 SCA1 chromosomes and identify a very late onset SCA1 family with a fully penetrant uninterrupted pathogenic allele containing 38 repeats. This finding supports the theory that polyQ toxicity is related to the increase of the length of the inherited tracts and not as previously hypothesized to the structural transition occurring above a specific threshold. In addition, the threshold of toxicity shifts to a shorter polyQ length with the increase of the lifespan in SCA1. Furthermore, we show that SCA1 intermediate alleles have a different behavior compared to the other polyglutamine disorders as they do not show reduced penetrance when uninterrupted. Therefore, the pathogenic mechanism in SCA1 is distinct from other cytosine-adenine-guanine (CAG) repeat disorders. Accurately sizing repeats is paramount in precision medicine and can be challenging particularly with borderline alleles. We examined plasmids containing cloned CAG repeat tracts alongside a triplet repeat primed polymerase chain reaction (TP PCR) CAG repeat ladder to improve accuracy in repeat sizing by fragment analysis. This method accurately sizes the repeats irrespective of repeat composition or length. We also improved the model for calculating repeat length from fragment analysis sizing by fragment analyzing 100 cloned repeats of known size. Therefore, we recommend these methods for accurately sizing repeat lengths and restriction enzyme digestion to identify interruptions for interpretation of a given allele's pathogenicity.
Keywords: CAG repeat; PolyQ; SCA1; ataxia; genetic counseling; neurodegeneration.
Figures


References
-
- Greenfield J. G. (1954). The Spino-Cerebellar Degenerations. Springfield, IL: Blackwell Scientific Publications.
LinkOut - more resources
Full Text Sources
Other Literature Sources

