Revisiting Netrin-1: One Who Guides (Axons)
- PMID: 30108487
- PMCID: PMC6080411
- DOI: 10.3389/fncel.2018.00221
Revisiting Netrin-1: One Who Guides (Axons)
Abstract
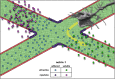
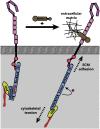
Proper patterning of the nervous system requires that developing axons find appropriate postsynaptic partners; this entails microns to meters of extension through an extracellular milieu exhibiting a wide range of mechanical and chemical properties. Thus, the elaborate networks of fiber tracts and non-fasciculated axons evident in mature organisms are formed via complex pathfinding. The macroscopic structures of axon projections are highly stereotyped across members of the same species, indicating precise mechanisms guide their formation. The developing axon exhibits directionally biased growth toward or away from external guidance cues. One of the most studied guidance cues is netrin-1, however, its presentation in vivo remains debated. Guidance cues can be secreted to form soluble or chemotactic gradients or presented bound to cells or the extracellular matrix to form haptotactic gradients. The growth cone, a highly specialized dynamic structure at the end of the extending axon, detects these guidance cues via transmembrane receptors, such as the netrin-1 receptors deleted in colorectal cancer (DCC) and UNC5. These receptors orchestrate remodeling of the cytoskeleton and cell membrane through both chemical and mechanotransductive pathways, which result in traction forces generated by the cytoskeleton against the extracellular environment and translocation of the growth cone. Through intracellular signaling responses, netrin-1 can trigger either attraction or repulsion of the axon. Here we review the mechanisms by which the classical guidance cue netrin-1 regulates intracellular effectors to respond to the extracellular environment in the context of axon guidance during development of the central nervous system and discuss recent findings that demonstrate the critical importance of mechanical forces in this process.
Keywords: DCC; UNC5; axon guidance; chemotaxis; growth cone; haptotaxis; netrin-1.
Figures







References
Publication types
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

