The Aβ Containing Brain Extracts Having Different Effects in Alzheimer's Disease Transgenic Caenorhabditis elegans and Mice
- PMID: 30108498
- PMCID: PMC6079246
- DOI: 10.3389/fnagi.2018.00208
The Aβ Containing Brain Extracts Having Different Effects in Alzheimer's Disease Transgenic Caenorhabditis elegans and Mice
Abstract
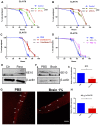
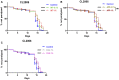
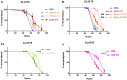
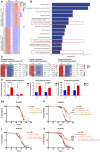
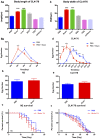
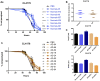
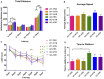
Background: The deposition of β-sheet rich amyloid in senile plaques is a pathological hallmark of Alzheimer's disease (AD), which is thought to cause neuronal dysfunction. Previous studies have strongly implicated that intracerebral infusion of brain extract containing aggregated β-amyloid (Aβ) is able to induce cerebral amyloidosis thus causing neuronal damage and clinical abnormalities in rodents and nonhuman primates, which are reminiscent of a prion-like mechanism. Prion disease has been documented in cases of prion-contaminated food consumption. Methods: We investigated whether cerebral transmission of Aβ was possible via oral administration of Aβ-rich brain extract in non-susceptible and susceptible host mice by immunohistochemistry, western blotting and behavior tests. Also brain extracts were supplied to AD transgenic Caenorhabditis elegans, and paralysis curve were conducted, following detection of Aβ amyloid. RNA sequencing of nematodes was applied then inhibitors for relevant dysregulated genes were used in the paralysis induction. Results: The oral treatment of AD brain extract or normal brain extract neither aggravated nor mitigated the Aβ load, glial activation or the abnormal behaviors in recipient Amyloid precursor protein/presenilin 1 (APP/PS1) mice. Whereas, a significant improvement of AD pathology was detected in worms treated with Aβ-rich or normal brain extracts, which was attributable to the heat-sensitive components of brain extracts. Transcriptome sequencing of CL4176 nematodes suggested that brain extracts could delay worm paralysis through multiple pathways, including ubiquitin mediated proteolysis and Transforming growth factor β (TGF-β) signaling pathway. Inhibitors of the ubiquitin proteasome system and the TGF-β signaling pathway significantly blocked the suppressive effects of brain extracts on worm paralysis. Conclusions: Our results suggest that systemic transmissible mechanisms of prion proteopathy may not apply to β amyloid, at least in terms of oral administration. However, brain extracts strongly ameliorated AD pathology in AD transgenic nematodes partially through TGF-β signaling pathway and ubiquitin mediated proteolysis, which indicated that some natural endogenous components in the mammalian tissues could resist Aβ toxicity.
Keywords: APP/PS1 transgenic mice; Alzheimer’s disease; RNA-sequencing (RNA-seq); oral administration; transgenic AD C. elegans; β-amyloid.
Figures

Similar articles
-
Cerebral inoculation of human A53T α-synuclein reduces spatial memory decline and amyloid-β aggregation in APP/PS1 transgenic mice of Alzheimer's disease.Brain Res Bull. 2018 Oct;143:116-122. doi: 10.1016/j.brainresbull.2018.10.003. Epub 2018 Oct 24. Brain Res Bull. 2018. PMID: 30366065
-
6-Methyluracil derivatives as acetylcholinesterase inhibitors for treatment of Alzheimer's disease.Int J Risk Saf Med. 2015;27 Suppl 1:S69-71. doi: 10.3233/JRS-150694. Int J Risk Saf Med. 2015. PMID: 26639718
-
Deer antler extracts reduce amyloid-beta toxicity in a Caenorhabditis elegans model of Alzheimer's disease.J Ethnopharmacol. 2022 Mar 1;285:114850. doi: 10.1016/j.jep.2021.114850. Epub 2021 Nov 19. J Ethnopharmacol. 2022. PMID: 34801608
-
Alzheimer's disease.Subcell Biochem. 2012;65:329-52. doi: 10.1007/978-94-007-5416-4_14. Subcell Biochem. 2012. PMID: 23225010 Review.
-
Impaired autophagy and APP processing in Alzheimer's disease: The potential role of Beclin 1 interactome.Prog Neurobiol. 2013 Jul-Aug;106-107:33-54. doi: 10.1016/j.pneurobio.2013.06.002. Epub 2013 Jul 1. Prog Neurobiol. 2013. PMID: 23827971 Review.
References
-
- Bian M., Yu M., Yang S., Gao H., Huang Y., Deng C., et al. (2008). Expression of Cbl-interacting protein of 85 kDa in MPTP mouse model of Parkinson’s disease and 1-methyl-4-phenyl-pyridinium ion-treated dopaminergic SH-SY5Y cells. Acta Biochim. Biophys. Sin. 40, 505–512. 10.1111/j.1745-7270.2008.00423.x - DOI - PubMed
LinkOut - more resources
Full Text Sources
Other Literature Sources

