Vascular Smooth Muscle Contractile Function Declines With Age in Skeletal Muscle Feed Arteries
- PMID: 30108507
- PMCID: PMC6079263
- DOI: 10.3389/fphys.2018.00856
Vascular Smooth Muscle Contractile Function Declines With Age in Skeletal Muscle Feed Arteries
Abstract
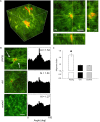
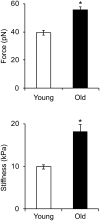
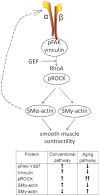
Aging induces a progressive decline in vasoconstrictor responses in central and peripheral arteries. This study investigated the hypothesis that vascular smooth muscle (VSM) contractile function declines with age in soleus muscle feed arteries (SFA). Contractile function of cannulated SFA isolated from young (4 months) and old (24 months) Fischer 344 rats was assessed by measuring constrictor responses of denuded (endothelium removed) SFA to norepinephrine (NE), phenylephrine (PE), and angiotensin II (Ang II). In addition, we investigated the role of RhoA signaling in modulation of VSM contractile function. Structural and functional characteristics of VSM cells were evaluated by fluorescence imaging and atomic force microscopy (AFM). Results indicated that constrictor responses to PE and Ang II were significantly impaired in old SFA, whereas constrictor responses to NE were preserved. In the presence of a Rho-kinase inhibitor (Y27632), constrictor responses to NE, Ang II, and PE were significantly reduced in young and old SFA. In addition, the age-group difference in constrictor responses to Ang II was eliminated. ROCK1 and ROCK2 content was similar in young and old VSM cells, whereas pROCK1 and pROCK2 were significantly elevated in old VSM cells. Aging was associated with a reduction in smooth muscle α-actin stress fibers and recruitment of proteins to cell-matrix adhesions. Old VSM cells presented an increase in integrin adhesion to the matrix and smooth muscle γ-actin fibers that was associated with increased cell stiffness. In conclusion, our results indicate that VSM contractile function declined with age in SFA. The decrement in contractile function was mediated in part by RhoA/ROCK signaling. Upregulation of pROCK in old VSM cells was not able to rescue contractility in old SFA. Collectively, these results indicate that changes at the VSM cell level play a central role in the reduced contractile function of aged SFA.
Keywords: Rho-kinase; aging; atomic force microscopy; vascular smooth muscle; vasoconstriction.
Figures



References
-
- Bai Y., Lee P. F., Gibbs H. C., Bayless K. J., Yeh A. T. (2014). Dynamic multicomponent engineered tissue reorganization and matrix deposition measured with an integrated nonlinear optical microscopy-optical coherence microscopy system. J. Biomed. Opt. 19:36014. 10.1117/1.JBO.19.3.036014 - DOI - PMC - PubMed
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

