Influence of the KCNQ1 S140G Mutation on Human Ventricular Arrhythmogenesis and Pumping Performance: Simulation Study
- PMID: 30108508
- PMCID: PMC6080549
- DOI: 10.3389/fphys.2018.00926
Influence of the KCNQ1 S140G Mutation on Human Ventricular Arrhythmogenesis and Pumping Performance: Simulation Study
Abstract
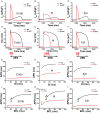
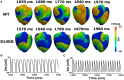
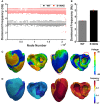
The KCNQ1 S140G mutation, which is involved in IKs current, affects atrial fibrillation. However, little is known about its effect on the mechanical behavior of the heart. Therefore, we assessed the influence of the KCNQ1 S140G mutation on ventricular electrophysiological stability and mechanical pumping performance using a multi-scale model of cardiac electromechanics. An image-based electromechanical model was used to assess the effect on electrical propagation and arrhythmogenesis of the KCNQ1 S140G mutation. In addition, it was used to compare the mechanical response under the wild-type (WT) and S140G mutation conditions. The intracellular calcium transient obtained from the electrophysiological model was applied as an input parameter to a mechanical model to implement excitation-contraction coupling. The IKs current equation was modified to account for expression of the KCNQ1 S140G mutation, and it included a scaling factor (ϕ) for mutant expressivity. The WT and S140G mutation conditions were compared at the single-cell and three-dimensional (3D) tissue levels. The action potential duration (APD) was reduced by 60% by the augmented IKs current under the S140G mutation condition, which resulted in shorter QT interval. This reduced the 3D sinus rhythm wavelength by 60% and the sustained re-entry by 56%. However, pumping efficiency of mutant ventricles was superior in sinus rhythm condition. In addition, the shortened wavelength in cardiac tissue allowed a re-entrant circuit to form and increased the probability of sustaining ventricular tachycardia and ventricular fibrillation. In contrast, under the WT condition, a normal wavelength (20.8 cm) was unlikely to initiate and sustain re-entry in the cardiac tissue. Subsequently, the S140G mutant ventricles developed a higher dominant frequency distribution range (2.0-5.3 Hz) than the WT condition (2.8-3.7 Hz). In addition, stroke volume of mutant ventricles was reduced by 65% in sustained re-entry compared to the WT condition. In conclusion, signs of the S140G mutation might be difficult to identify in sinus rhythm even though the mutant ventricles show shortened QT interval. This suggests that the KCNQ1 S140G mutation increases the risk of death by sudden cardiac arrest. In addition, the KCNQ1 S140G mutation can induce ventricular arrhythmia and lessen ventricular contractility under re-entrant conditions.
Keywords: KCNQ1 S140G mutation; dominant frequency; electromechanical simulation; pumping performance; reentry response; sinus rhythm response; ventricular arrhythmia.
Figures





Similar articles
-
Computational prediction of the effect of D172N KCNJ2 mutation on ventricular pumping during sinus rhythm and reentry.Med Biol Eng Comput. 2020 May;58(5):977-990. doi: 10.1007/s11517-020-02124-w. Epub 2020 Feb 24. Med Biol Eng Comput. 2020. PMID: 32095980
-
Pro-arrhythmogenic effects of the S140G KCNQ1 mutation in human atrial fibrillation - insights from modelling.J Physiol. 2012 Sep 15;590(18):4501-14. doi: 10.1113/jphysiol.2012.229146. Epub 2012 Apr 16. J Physiol. 2012. PMID: 22508963 Free PMC article.
-
The S140G KCNQ1 atrial fibrillation mutation affects 'I(KS)' profile during both atrial and ventricular action potentials.J Physiol Pharmacol. 2010 Dec;61(6):759-64. J Physiol Pharmacol. 2010. PMID: 21224508
-
Selective targeting of gain-of-function KCNQ1 mutations predisposing to atrial fibrillation.Circ Arrhythm Electrophysiol. 2013 Oct;6(5):960-6. doi: 10.1161/CIRCEP.113.000439. Epub 2013 Sep 4. Circ Arrhythm Electrophysiol. 2013. PMID: 24006450 Free PMC article.
-
Computational Study to Identify the Effects of the KCNJ2 E299V Mutation in Cardiac Pumping Capacity.Comput Math Methods Med. 2020 Mar 31;2020:7194275. doi: 10.1155/2020/7194275. eCollection 2020. Comput Math Methods Med. 2020. PMID: 32328155 Free PMC article.
Cited by
-
Proarrhythmogenic Effect of the L532P and N588K KCNH2 Mutations in the Human Heart Using a 3D Electrophysiological Model.J Korean Med Sci. 2020 Jul 27;35(29):e238. doi: 10.3346/jkms.2020.35.e238. J Korean Med Sci. 2020. PMID: 32715669 Free PMC article.
-
Relationship Between Electrical Instability and Pumping Performance During Ventricular Tachyarrhythmia: Computational Study.Front Physiol. 2020 Mar 24;11:220. doi: 10.3389/fphys.2020.00220. eCollection 2020. Front Physiol. 2020. PMID: 32265731 Free PMC article.
-
Prediction of the mechanical response of cardiac alternans by using an electromechanical model of human ventricular myocytes.Biomed Eng Online. 2019 Jun 7;18(1):72. doi: 10.1186/s12938-019-0690-x. Biomed Eng Online. 2019. PMID: 31174533 Free PMC article.
-
Computational Prediction of the Combined Effect of CRT and LVAD on Cardiac Electromechanical Delay in LBBB and RBBB.Comput Math Methods Med. 2018 Nov 14;2018:4253928. doi: 10.1155/2018/4253928. eCollection 2018. Comput Math Methods Med. 2018. PMID: 30538769 Free PMC article.
-
Computational prediction of the effect of D172N KCNJ2 mutation on ventricular pumping during sinus rhythm and reentry.Med Biol Eng Comput. 2020 May;58(5):977-990. doi: 10.1007/s11517-020-02124-w. Epub 2020 Feb 24. Med Biol Eng Comput. 2020. PMID: 32095980
References
-
- El Harchi A., Zhang H., Hancox J. (2010). The S140G KCNQ1 atrial fibrillation mutation affects ‘I (KS)'profile during both atrial and ventricular action potentials. J. Physiol. Pharmacol. 61, 759–764. - PubMed
LinkOut - more resources
Full Text Sources
Other Literature Sources

