Involvement of TRPV1 Channels in Energy Homeostasis
- PMID: 30108548
- PMCID: PMC6079260
- DOI: 10.3389/fendo.2018.00420
Involvement of TRPV1 Channels in Energy Homeostasis
Abstract
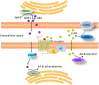
The ion channel TRPV1 is involved in a wide range of processes including nociception, thermosensation and, more recently discovered, energy homeostasis. Tightly controlling energy homeostasis is important to maintain a healthy body weight, or to aid in weight loss by expending more energy than energy intake. TRPV1 may be involved in energy homeostasis, both in the control of food intake and energy expenditure. In the periphery, it is possible that TRPV1 can impact on appetite through control of appetite hormone levels or via modulation of gastrointestinal vagal afferent signaling. Further, TRPV1 may increase energy expenditure via heat production. Dietary supplementation with TRPV1 agonists, such as capsaicin, has yielded conflicting results with some studies indicating a reduction in food intake and increase in energy expenditure, and other studies indicating the converse. Nonetheless, it is increasingly apparent that TRPV1 may be dysregulated in obesity and contributing to the development of this disease. The mechanisms behind this dysregulation are currently unknown but interactions with other systems, such as the endocannabinoid systems, could be altered and therefore play a role in this dysregulation. Further, TRPV1 channels appear to be involved in pancreatic insulin secretion. Therefore, given its plausible involvement in regulation of energy and glucose homeostasis and its dysregulation in obesity, TRPV1 may be a target for weight loss therapy and diabetes. However, further research is required too fully elucidate TRPV1s role in these processes. The review provides an overview of current knowledge in this field and potential areas for development.
Keywords: TRPV1; appetite regulation; endocannabinoid; endovanilloid; metabolism; obesity.
Figures




References
Publication types
LinkOut - more resources
Full Text Sources
Other Literature Sources

