Mixotrophy in Chlorophytes and Haptophytes-Effect of Irradiance, Macronutrient, Micronutrient and Vitamin Limitation
- PMID: 30108563
- PMCID: PMC6080504
- DOI: 10.3389/fmicb.2018.01704
Mixotrophy in Chlorophytes and Haptophytes-Effect of Irradiance, Macronutrient, Micronutrient and Vitamin Limitation
Abstract
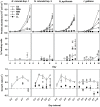
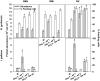
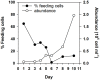
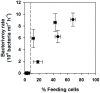
Chlorophytes and haptophytes are key contributors to global phytoplankton biomass and productivity. Mixotrophic bacterivory has been detected for both groups, but a shortage of studies with cultured representatives hinders a consistent picture of the ecological relevance and regulation of this trophic strategy. Here, the growth, primary production, fraction of feeding cells (acidotropic probes) and bacterivory rates (surrogate prey) are tested for two species of the chlorophyte genus Nephroselmis and the haptophyte Isochrysis galbana under contrasting regimes of light (high vs. low) and nutrients (non-limited and macronutrient-, micronutrient- and vitamin-limited), at low bacterial concentrations (<107 bacteria mL-1). All three species were obligate phototrophs, unable to compensate for low light conditions through feeding. Under nutrient limitation, N. rotunda and I. galbana fed, but growth ceased or was significantly lower than in the control. Thus, mixotrophic bacterivory could be a survival rather than a growth strategy for certain species. In contrast, nutrient-limited N. pyriformis achieved growth rates equivalent to the control through feeding. This strikingly differs with the classical view of chlorophytes as primarily non-feeders and indicates mixotrophic bacterivory can be a significant trophic strategy for green algae, even at the low bacterial concentrations found in oligotrophic open oceans.
Keywords: bacterivory; chlorophyte; green algae; haptophyte; mixotrophic growth; mixotrophy; phytoflagellate.
Figures
References
-
- Aaronson S. (1974). The biology and ultrastructure of phagotrophy in Ochromonas danica (Chrysophyceae: Chrysomonadida). J. Gen. Microbiol. 83, 21–29. 10.1099/00221287-83-1-21 - DOI
-
- Bell E. M., Laybourn-Parry J. (2003). Mixotrophy in the Antartic phytoflagellate Pyramimonas gelidicola (Chlorophyta: Prasinophyceae). J. Phycol. 649, 644–649. 10.1046/j.1529-8817.2003.02152.x - DOI
-
- Berge T., Hansen P. J., Moestrup Ø. (2008). Feeding mechanism, prey specificity and growth in light and dark of the plastidic dinoflagellate Karlodinium armiger. Aquat. Microb. Ecol. 50, 279–288. 10.3354/ame01165 - DOI
LinkOut - more resources
Full Text Sources
Other Literature Sources

