CL-L1 and CL-K1 Exhibit Widespread Tissue Distribution With High and Co-Localized Expression in Secretory Epithelia and Mucosa
- PMID: 30108587
- PMCID: PMC6079254
- DOI: 10.3389/fimmu.2018.01757
CL-L1 and CL-K1 Exhibit Widespread Tissue Distribution With High and Co-Localized Expression in Secretory Epithelia and Mucosa
Abstract
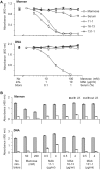
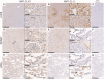
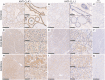
Collectin liver 1 (CL-L1, alias collectin 10) and collectin kidney 1 (CL-K1, alias collectin 11) are oligomeric pattern recognition molecules associated with the complement system, and mutations in either of their genes may lead to deficiency and developmental defects. The two collectins are reportedly localized and synthesized in the liver, kidneys, and adrenals, and can be found in the circulation as heteromeric complexes (CL-LK), which upon binding to microbial high mannose-like glycoconjugates activates the complement system via the lectin activation pathway. The tissue distribution of homo- vs. heteromeric CL-L1 and -K1 complexes, the mechanism of heteromeric complex formation and in which tissues this occurs, is hitherto incompletely described. We have by immunohistochemistry using monoclonal antibodies addressed the precise cellular localization of the two collectins in the main human tissues. We find that the two collectins have widespread and almost identical tissue distribution with a high expression in epithelial cells in endo-/exocrine secretory tissues and mucosa. There is also accordance between localization of mRNA transcripts and detection of proteins, showing that local synthesis likely is responsible for peripheral localization and eventual formation of the CL-LK complexes. The functional implications of the high expression in endo-/exocrine secretory tissue and mucosa is unknown but might be associated with the activity of MASP-3, which has a similar pattern of expression and is known to potentiate the activity of the alternative complement activation pathway.
Keywords: 3MC syndrome; collectin; complement system; innate immunity; mucosal immunology.
Figures




Similar articles
-
Novel Monoclonal Antibodies Against Native Human Collectin L1, Using the Heteromeric Complex CL-LK as Immunogen.Monoclon Antib Immunodiagn Immunother. 2018 Nov;37(5):207-211. doi: 10.1089/mab.2018.0024. Monoclon Antib Immunodiagn Immunother. 2018. PMID: 30362927
-
The collectins CL-L1, CL-K1 and CL-P1, and their roles in complement and innate immunity.Immunobiology. 2016 Oct;221(10):1058-67. doi: 10.1016/j.imbio.2016.05.012. Epub 2016 Jun 2. Immunobiology. 2016. PMID: 27377710 Review.
-
Heteromeric complexes of native collectin kidney 1 and collectin liver 1 are found in the circulation with MASPs and activate the complement system.J Immunol. 2013 Dec 15;191(12):6117-27. doi: 10.4049/jimmunol.1302121. Epub 2013 Oct 30. J Immunol. 2013. PMID: 24174618
-
Structure and function of collectin liver 1 (CL-L1) and collectin 11 (CL-11, CL-K1).Immunobiology. 2012 Sep;217(9):851-63. doi: 10.1016/j.imbio.2011.12.008. Epub 2012 Feb 4. Immunobiology. 2012. PMID: 22475410 Review.
-
Collectin 11 (CL-11, CL-K1) is a MASP-1/3-associated plasma collectin with microbial-binding activity.J Immunol. 2010 Nov 15;185(10):6096-104. doi: 10.4049/jimmunol.1002185. Epub 2010 Oct 18. J Immunol. 2010. PMID: 20956340
Cited by
-
Absence of the Lectin Activation Pathway of Complement Ameliorates Proteinuria-Induced Renal Injury.Front Immunol. 2019 Sep 23;10:2238. doi: 10.3389/fimmu.2019.02238. eCollection 2019. Front Immunol. 2019. PMID: 31608060 Free PMC article.
-
Serum Proteomic Analysis by Tandem Mass Tag-Based Quantitative Proteomics in Pediatric Obstructive Sleep Apnea.Front Mol Biosci. 2022 Apr 11;9:762336. doi: 10.3389/fmolb.2022.762336. eCollection 2022. Front Mol Biosci. 2022. PMID: 35480887 Free PMC article.
-
Rationale for targeting complement in COVID-19.EMBO Mol Med. 2020 Aug 7;12(8):e12642. doi: 10.15252/emmm.202012642. Epub 2020 Jul 12. EMBO Mol Med. 2020. PMID: 32559343 Free PMC article. Review.
-
Clinical associations of complement-activating collectins, collectin-10, collectin-11 and mannose-binding lectin in preterm neonates.Front Immunol. 2024 Oct 11;15:1463651. doi: 10.3389/fimmu.2024.1463651. eCollection 2024. Front Immunol. 2024. PMID: 39464884 Free PMC article.
-
A Plausible Role for Collectins in Skin Immune Homeostasis.Front Immunol. 2021 Mar 15;12:594858. doi: 10.3389/fimmu.2021.594858. eCollection 2021. Front Immunol. 2021. PMID: 33790889 Free PMC article. Review.
References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Miscellaneous

