Interleukin-33 Contributes to the Induction of Th9 Cells and Antitumor Efficacy by Dectin-1-Activated Dendritic Cells
- PMID: 30108595
- PMCID: PMC6079242
- DOI: 10.3389/fimmu.2018.01787
Interleukin-33 Contributes to the Induction of Th9 Cells and Antitumor Efficacy by Dectin-1-Activated Dendritic Cells
Abstract
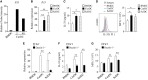
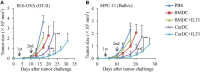
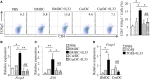
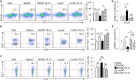
We recently discovered that dectin-1-activated dendritic cells (DCs) drive potent T helper (Th) 9 cell responses and antitumor immunity. However, the underlying mechanisms need to be further defined. The cytokine microenvironment is critical for Th cell differentiation. Here, we show that dectin-1 activation enhances interleukin (IL)-33 expression in DCs. We found that blocking IL-33/ST2 inhibits dectin-1-activated DC-induced Th9 cell differentiation. More importantly, the addition of IL-33 further promotes Th9 cell priming and antitumor efficacy induced by dectin-1-activated DCs. Mechanistically, in addition to the promotion of Th9 and Th1 cells, dectin-1-activated DCs combined with IL-33 abolish the activity of IL-33 in the induction of regulatory T cells. Furthermore, the combined treatment of dectin-1-activated DCs and IL-33 increases the frequencies of CD4+ T cells by fostering their proliferation and inhibiting their exhaustive differentiation. Thus, our results demonstrate the important role of IL-33 in dectin-1-activated DC-induced Th9 cell differentiation and antitumor efficacy, and suggest that the combination of dectin-1-activated DCs and IL-33 may present a new effective modality of DC-based vaccines in tumor immunotherapy.
Keywords: Th9; cancer immunotherapy; dectin-1; dendritic cells; interleukin-33.
Figures


Similar articles
-
Dectin-1-activated dendritic cells trigger potent antitumour immunity through the induction of Th9 cells.Nat Commun. 2016 Aug 5;7:12368. doi: 10.1038/ncomms12368. Nat Commun. 2016. PMID: 27492902 Free PMC article.
-
Dectin-1 stimulates IL-33 expression in dendritic cells via upregulation of IRF4.Lab Invest. 2018 Jun;98(6):708-714. doi: 10.1038/s41374-018-0047-2. Epub 2018 Mar 14. Lab Invest. 2018. PMID: 29540860
-
IL-33 drives the antitumor effects of dendritic cells via the induction of Tc9 cells.Cell Mol Immunol. 2019 Jul;16(7):644-651. doi: 10.1038/s41423-018-0166-0. Epub 2018 Oct 1. Cell Mol Immunol. 2019. PMID: 30275536 Free PMC article.
-
IL-9 and Th9 Cells in Tumor Immunity.Adv Exp Med Biol. 2020;1240:35-46. doi: 10.1007/978-3-030-38315-2_3. Adv Exp Med Biol. 2020. PMID: 32060886 Review.
-
IL-33 and IL-33-derived DC-based tumor immunotherapy.Exp Mol Med. 2024 Jun;56(6):1340-1347. doi: 10.1038/s12276-024-01249-4. Epub 2024 Jun 3. Exp Mol Med. 2024. PMID: 38825642 Free PMC article. Review.
Cited by
-
IL-24 is the key effector of Th9 cell-mediated tumor immunotherapy.iScience. 2023 Aug 3;26(9):107531. doi: 10.1016/j.isci.2023.107531. eCollection 2023 Sep 15. iScience. 2023. PMID: 37680459 Free PMC article.
-
The Contradictory Role of Interleukin-33 in Immune Cells and Tumor Immunity.Cancer Manag Res. 2020 Aug 21;12:7527-7537. doi: 10.2147/CMAR.S262745. eCollection 2020. Cancer Manag Res. 2020. PMID: 32904627 Free PMC article. Review.
-
Activin A Promotes Differentiation of a Pathogenic Multicytokine IL-9-secreting CD4+ T Cell Population.J Immunol. 2024 Sep 15;213(6):823-830. doi: 10.4049/jimmunol.2300635. J Immunol. 2024. PMID: 39058312
-
Targeting the Epithelium-Derived Innate Cytokines: From Bench to Bedside.Immune Netw. 2022 Feb 22;22(1):e11. doi: 10.4110/in.2022.22.e11. eCollection 2022 Feb. Immune Netw. 2022. PMID: 35291657 Free PMC article. Review.
-
Association of IL-9 Cytokines with Hepatic Injury in Echinococcus granulosus Infection.Biomolecules. 2024 Aug 14;14(8):1007. doi: 10.3390/biom14081007. Biomolecules. 2024. PMID: 39199394 Free PMC article.
References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials

