Desmoplasia in pancreatic ductal adenocarcinoma: insight into pathological function and therapeutic potential
- PMID: 30108679
- PMCID: PMC6086006
- DOI: 10.18632/genesandcancer.171
Desmoplasia in pancreatic ductal adenocarcinoma: insight into pathological function and therapeutic potential
Abstract
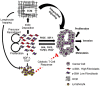
Extensive desmoplasia is a prominent feature of the pancreatic ductal adenocarcinoma (PDAC) microenvironment. Initially, studies demonstrated that desmoplasia promotes proliferation, invasion and chemoresistance in PDAC cells. While these findings suggested the therapeutic potential of targeting desmoplasia in PDAC, more recent studies utilizing genetically-engineered mouse models of PDAC, which lack key components of desmoplasia, demonstrated accelerated progression of PDAC. This contrast calls into question the paradigm that desmoplasia unilaterally promotes PDAC progression and the premise of desmoplasia-targeted therapy. This review briefly examines the major reports of the tumor-promoting and -restraining roles of desmoplasia in PDAC with commentary on the gaps in our current understanding of desmoplasia in PDAC. Additionally, we discuss the studies demonstrating the heterogeneous and multifaceted nature of desmoplasia in PDAC and advocate for future areas of research to thoroughly address the various facets of desmoplasia in PDAC, reconcile seemingly contradictory reports of the role of desmoplasia in PDAC progression, and discover aspects of desmoplasia that are therapeutically actionable.
Keywords: SHH; cancer-associated fibroblast; desmoplasia; extracellular matrix; pancreatic ductal adenocarcinoma.
Conflict of interest statement
CONFLICTS OF INTEREST The authors have no conflicts of interest to declare.
Figures

References
-
- Hall Bradley R., Cannon Andrew, Atri Pranita, Wichman Christopher S., Smith Lynette M., Ganti Apar K., Are Chandrakanth, Sasson Aaron, Kumar Sushil, Batra Surinder K. Advanced pancreatic cancer: a meta-analysis of clinical trials over thirty years. Oncotarget. 2018 ePub ahead of Print. - PMC - PubMed
-
- Sherman MH, Yu RT, Engle DD, Ding N, Atkins AR, Tiriac H, Collisson EA, Connor F, Van Dyke T, Kozlov S, Martin P, Tseng TW, Dawson DW, et al. Vitamin D receptor-mediated stromal reprogramming suppresses pancreatitis and enhances pancreatic cancer therapy. Cell. 2014;159:80–93. doi: 10.1016/j.cell.2014.08.007. - DOI - PMC - PubMed
-
- Carapuca EF, Gemenetzidis E, Feig C, Bapiro TE, Williams MD, Wilson AS, Delvecchio FR, Arumugam P, Grose RP, Lemoine NR, Richards FM, Kocher HM. Anti-stromal treatment together with chemotherapy targets multiple signalling pathways in pancreatic adenocarcinoma. J Pathol. 2016;239:286–96. doi: 10.1002/path.4727. - DOI - PMC - PubMed
-
- Ene-Obong A, Clear AJ, Watt J, Wang J, Fatah R, Riches JC, Marshall JF, Chin-Aleong J, Chelala C, Gribben JG, Ramsay AG, Kocher HM. Activated pancreatic stellate cells sequester CD8+ T cells to reduce their infiltration of the juxtatumoral compartment of pancreatic ductal adenocarcinoma. Gastroenterology. 2013;145:1121–32. doi: 10.1053/j.gastro.2013.07.025. - DOI - PMC - PubMed
Publication types
Grants and funding
- R01 CA206444/CA/NCI NIH HHS/United States
- U01 CA200466/CA/NCI NIH HHS/United States
- R01 CA195586/CA/NCI NIH HHS/United States
- U01 CA213862/CA/NCI NIH HHS/United States
- P50 CA127297/CA/NCI NIH HHS/United States
- R01 CA201444/CA/NCI NIH HHS/United States
- R01 GM113166/GM/NIGMS NIH HHS/United States
- R44 DK117472/DK/NIDDK NIH HHS/United States
- U01 CA210240/CA/NCI NIH HHS/United States
- R01 CA183459/CA/NCI NIH HHS/United States
- R01 CA210637/CA/NCI NIH HHS/United States
- F30 CA225117/CA/NCI NIH HHS/United States
- R21 AA026428/AA/NIAAA NIH HHS/United States
LinkOut - more resources
Full Text Sources
Other Literature Sources

