Design, synthesis, anticancer activity and docking studies of theophylline containing 1,2,3-triazoles with variant amide derivatives
- PMID: 30108703
- PMCID: PMC6072493
- DOI: 10.1039/c6md00479b
Design, synthesis, anticancer activity and docking studies of theophylline containing 1,2,3-triazoles with variant amide derivatives
Abstract
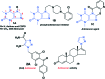
A new series of theophylline analogues containing 1,2,3-triazoles with different amide groups (22-41) has been designed and synthesized, and their biological activities have been evaluated as potential anticancer agents. The anticancer activities of the synthesized compounds were studied in four cancer cell lines viz. lung (A549), colon (HT-29), breast (MCF-7) and melanoma (A375). Furthermore, these compounds were screened for computational ADME and Lipinski's analysis followed by molecular docking and binding energy calculations against the various therapeutic targets involved in cell proliferation. The in vitro results demonstrate that compounds 22, 27, 36 and 40 have pivotal anticancer activity. Among these, compounds 22 and 27 have significant cytotoxic activity in all three cell lines; the in silico docking studies also reveal that compounds 22, 27 and 36 have good dock scores, binding affinities and binding energies towards human epidermal growth factor receptor 2. This is the first report to demonstrate theophylline hybrids containing 1,2,3-triazoles as potential anticancer agents.
Figures


Similar articles
-
Design, synthesis, anticancer, antimicrobial activities and molecular docking studies of theophylline containing acetylenes and theophylline containing 1,2,3-triazoles with variant nucleoside derivatives.Eur J Med Chem. 2016 Nov 10;123:379-396. doi: 10.1016/j.ejmech.2016.07.024. Epub 2016 Jul 15. Eur J Med Chem. 2016. PMID: 27487568
-
Design, Synthesis, In Vitro Anti-cancer Activity, ADMET Profile and Molecular Docking of Novel Triazolo[3,4-a]phthalazine Derivatives Targeting VEGFR-2 Enzyme.Anticancer Agents Med Chem. 2018;18(8):1184-1196. doi: 10.2174/1871520618666180412123833. Anticancer Agents Med Chem. 2018. PMID: 29651967
-
Hydrazide-hydrazones as Small Molecule Tropomyosin Receptor Kina se A (TRKA) Inhibitors: Synthesis, Anticancer Activities, In silico ADME and Molecular Docking Studies.Med Chem. 2022;19(1):47-63. doi: 10.2174/1573406418666220427105041. Med Chem. 2022. PMID: 35490310
-
Computational and Molecular Docking Studies of New Benzene Sulfonamide Drugs with Anticancer and Antioxidant Effects.Curr Org Synth. 2023;20(3):339-350. doi: 10.2174/1570179420666221007141937. Curr Org Synth. 2023. PMID: 36214306
-
Chemical Characterization, In-silico Evaluation, and Molecular Docking Analysis of Antiproliferative Compounds Isolated from the Bark of Anthocephalus cadamba Miq.Anticancer Agents Med Chem. 2022;22(20):3416-3437. doi: 10.2174/1871520622666220204123348. Anticancer Agents Med Chem. 2022. PMID: 35125087
Cited by
-
Antitumor evaluation of novel phenothiazine derivatives that inhibit migration and tubulin polymerization against gastric cancer MGC-803 cells.Invest New Drugs. 2019 Feb;37(1):188-198. doi: 10.1007/s10637-018-0682-x. Epub 2018 Oct 22. Invest New Drugs. 2019. PMID: 30345465
-
Design, Synthesis, and the Biological Evaluation of a New Series of Acyclic 1,2,3-Triazole Nucleosides.Arch Pharm (Weinheim). 2017 Sep;350(9):1700166. doi: 10.1002/ardp.201700166. Epub 2017 Aug 1. Arch Pharm (Weinheim). 2017. PMID: 28763115 Free PMC article.
-
Adsorption and corrosion inhibition behaviour of new theophylline-triazole-based derivatives for steel in acidic medium.R Soc Open Sci. 2019 Mar 27;6(3):181738. doi: 10.1098/rsos.181738. eCollection 2019 Mar. R Soc Open Sci. 2019. PMID: 31032030 Free PMC article.
-
1,2,3-Triazole-Containing Compounds as Anti-Lung Cancer Agents: Current Developments, Mechanisms of Action, and Structure-Activity Relationship.Front Pharmacol. 2021 Jun 11;12:661173. doi: 10.3389/fphar.2021.661173. eCollection 2021. Front Pharmacol. 2021. PMID: 34177578 Free PMC article. Review.
-
Molecular Hybridization of Alkaloids Using 1,2,3-Triazole-Based Click Chemistry.Molecules. 2023 Nov 14;28(22):7593. doi: 10.3390/molecules28227593. Molecules. 2023. PMID: 38005315 Free PMC article. Review.
References
-
- World Health Statistics 2012, World Health Organization, Geneva, 2012.
-
- Dikshit R., Gupta P. C., Ramasundarahettige C., Gajalakshmi V., Aleksandrowicz L., Badwe R., Kumar R., Roy S., Suraweera W., Bray F., Mallath M., Singh P. K., Sinha D. N., Shet A. S., Gelband H., Jha P. Lancet. 2012;379:1807–1816. - PubMed
-
- National Cancer Institute, Retrieved, 15 July 2014.
-
- World Cancer Report, International Agency for Research on Cancer, 2008, Retrieved, 26 February 2011.
-
- Cancer Survival in England: Patients Diagnosed (2007–2011) and Followed up to 2012, Office for National Statistics, 29 October 2013, Retrieved, 29 June 2014.
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials

