Benzisoxazole: a privileged scaffold for medicinal chemistry
- PMID: 30108720
- PMCID: PMC6072331
- DOI: 10.1039/c7md00449d
Benzisoxazole: a privileged scaffold for medicinal chemistry
Abstract
The benzisoxazole analogs represent one of the privileged structures in medicinal chemistry and there has been an increasing number of studies on benzisoxazole-containing compounds. The unique benzisoxazole scaffold also exhibits an impressive potential as antimicrobial, anticancer, anti-inflammatory, anti-glycation agents and so on. This review examines the state of the art in medicinal chemistry as it relates to the comprehensive and general summary of the different benzisoxazole analogs, their use as starting building blocks of multifarious architectures on scales sufficient to drive human drug trials. The number of reports describing benzisoxazole-containing highly active compounds leads to the expectation that this scaffold will further emerge as a potential candidate in the field of drug discovery.
Figures



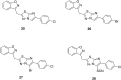

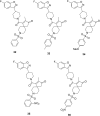
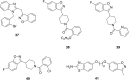
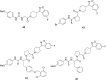




References
-
- Bioactive Heterocycles II, Topics in Heterocyclic Chemistry, Eguchi S., 2007, vol. 8, XII, p. 249.
-
- Gomtsyan A. Chem. Heterocycl. Compd. 2012;48:7–10.
-
- Kirk K. L. and Filler R., In Biomedical Frontiers of Fluorine Chemistry, Symposium Series, American Chemical Society, Washington, DC, 1996, vol. 639, pp. 1–24.
-
- Gelders Y. G., Heylen S. L. E., Vander B. G., Reyntjens A. J. M., Janssen P. A. J. Pharmacopsychiatry. 1990;23:206–211. - PubMed
-
- Dollery C., Therapeutic drugs, Churchill Livingstone, Edinburgh, UK, 1999.
Publication types
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

