Synergism of fused bicyclic 2-aminothiazolyl compounds with polymyxin B against Klebsiella pneumoniae
- PMID: 30108723
- PMCID: PMC6071964
- DOI: 10.1039/c7md00354d
Synergism of fused bicyclic 2-aminothiazolyl compounds with polymyxin B against Klebsiella pneumoniae
Abstract
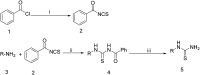
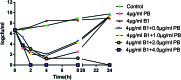
A series of fused bicyclic 2-aminothiazolyl compounds were synthesized and evaluated for their synergistic effects with polymyxin B (PB) against Klebsiella pneumoniae (SIPI-KPN-1712). Some of the synthesized compounds exhibited synergistic activity. When 4 μg ml-1 compound B1 was combined with PB, it showed potent antibacterial activity, achieving 64-fold reduction of the MIC of PB. Furthermore, compound B1 showed prominent synergistic efficacy in both concentration gradient and time-kill curves in vitro. In addition, B1 combined with PB also exhibited synergistic and partial synergistic effect against E. coli (ATCC25922 and its clinical isolates), Acinetobacter baumannii (ATCC19606 and its clinical isolates), and Pseudomonas aeruginosa (Pae-1399).
Figures

Similar articles
-
From Breast Cancer to Antimicrobial: Combating Extremely Resistant Gram-Negative "Superbugs" Using Novel Combinations of Polymyxin B with Selective Estrogen Receptor Modulators.Microb Drug Resist. 2017 Jul;23(5):640-650. doi: 10.1089/mdr.2016.0196. Epub 2016 Dec 9. Microb Drug Resist. 2017. PMID: 27935770
-
Synergism of eravacycline combined with other antimicrobial agents against carbapenem-resistant Enterobacteriaceae and Acinetobacter baumannii.J Glob Antimicrob Resist. 2022 Sep;30:56-59. doi: 10.1016/j.jgar.2022.05.020. Epub 2022 May 31. J Glob Antimicrob Resist. 2022. PMID: 35660472
-
Activity of ceftazidime-avibactam alone and in combination with polymyxin B against carbapenem-resistant Klebsiella pneumoniae in a tandem in vitro time-kill/in vivo Galleria mellonella survival model analysis.Int J Antimicrob Agents. 2020 Jan;55(1):105852. doi: 10.1016/j.ijantimicag.2019.11.009. Epub 2019 Nov 23. Int J Antimicrob Agents. 2020. PMID: 31770627
-
Synergistic combinations of polymyxins.Int J Antimicrob Agents. 2016 Dec;48(6):607-613. doi: 10.1016/j.ijantimicag.2016.09.014. Epub 2016 Oct 24. Int J Antimicrob Agents. 2016. PMID: 27865626 Free PMC article. Review.
-
Polymyxin combination therapy for multidrug-resistant, extensively-drug resistant, and difficult-to-treat drug-resistant gram-negative infections: is it superior to polymyxin monotherapy?Expert Rev Anti Infect Ther. 2023 Apr;21(4):387-429. doi: 10.1080/14787210.2023.2184346. Epub 2023 Mar 8. Expert Rev Anti Infect Ther. 2023. PMID: 36820511 Review.
Cited by
-
Study of Benzofuroquinolinium Derivatives as a New Class of Potent Antibacterial Agent and the Mode of Inhibition Targeting FtsZ.Front Microbiol. 2018 Aug 17;9:1937. doi: 10.3389/fmicb.2018.01937. eCollection 2018. Front Microbiol. 2018. PMID: 30174667 Free PMC article.
References
-
- Pendleton J. N., Gorman S. P., Gilmore B. F. Expert Rev. Anti-Infect. Ther. 2013;11:297–308. - PubMed
-
- Dosler S., Karaaslan E., Alev G. A. Aust. J. Chem. 2016;28:95–103. - PubMed
-
- Vaara M., Siikanen O., Apajalahti J., Frimodt-Møller N., Vaara T. J. Antimicrob. Chemother. 2010;65:942–945. - PubMed
-
- Gopu V., Meena C. K., Murali A., Shetty P. H. RSC Adv. 2015;6:2592–2601.
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

