Triazolopyrimidines identified as reversible myeloperoxidase inhibitors
- PMID: 30108726
- PMCID: PMC6071758
- DOI: 10.1039/c7md00268h
Triazolopyrimidines identified as reversible myeloperoxidase inhibitors
Abstract
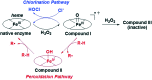
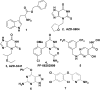
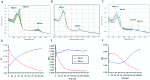
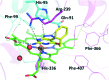
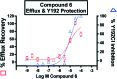
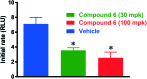
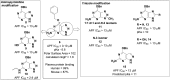
Myeloperoxidase, a mammalian peroxidase involved in the immune system as an anti-microbial first responder, can produce hypochlorous acid in response to invading pathogens. Myeloperoxidase has been implicated in several chronic pathological diseases due to the chronic production of hypochlorous acid, as well as other reactive radical species. A high throughput screen and triaging protocol was developed to identify a reversible inhibitor of myeloperoxidase toward the potential treatment of chronic diseases such as atherosclerosis. The identification and characterization of a reversible myeloperoxidase inhibitor, 7-(benzyloxy)-3H-[1,2,3]triazolo[4,5-d]pyrimidin-5-amine is described.
Figures

Similar articles
-
Inhibition of Myeloperoxidase.Handb Exp Pharmacol. 2021;264:261-285. doi: 10.1007/164_2020_388. Handb Exp Pharmacol. 2021. PMID: 33372235
-
Differential effects of oxidizing agents on human plasma alpha 1-proteinase inhibitor and human neutrophil myeloperoxidase.Biochemistry. 1985 Apr 9;24(8):1941-5. doi: 10.1021/bi00329a021. Biochemistry. 1985. PMID: 2990544
-
A novel strategy for evaluation of natural products acting on the myeloperoxidase/hypochlorous acid system by combining high-performance liquid chromatography-photodiode array detection-chemiluminescence and ultrafiltration-mass spectrometry techniques.J Sep Sci. 2018 Nov;41(22):4222-4232. doi: 10.1002/jssc.201800658. Epub 2018 Nov 2. J Sep Sci. 2018. PMID: 30194713
-
Biomarkers of myeloperoxidase-derived hypochlorous acid.Free Radic Biol Med. 2000 Sep 1;29(5):403-9. doi: 10.1016/s0891-5849(00)00204-5. Free Radic Biol Med. 2000. PMID: 11020661 Review.
-
Comparative reactivity of myeloperoxidase-derived oxidants with mammalian cells.Free Radic Biol Med. 2014 Jun;71:240-255. doi: 10.1016/j.freeradbiomed.2014.03.004. Epub 2014 Mar 13. Free Radic Biol Med. 2014. PMID: 24632382 Review.
Cited by
-
Computational assessment of the primary and secondary antioxidant potential of alkylresorcinols in physiological media.RSC Adv. 2023 Oct 9;13(42):29463-29476. doi: 10.1039/d3ra05967g. eCollection 2023 Oct 4. RSC Adv. 2023. PMID: 37818267 Free PMC article.
-
A Photoelectrochemical Sensor for the Detection of Hypochlorous Acid with a Phenothiazine-Based Photosensitizer.Molecules. 2024 Jan 27;29(3):614. doi: 10.3390/molecules29030614. Molecules. 2024. PMID: 38338358 Free PMC article.
-
Targeting Myeloperoxidase Ameliorates Gouty Arthritis: A Virtual Screening Success Story.J Med Chem. 2024 Jul 25;67(14):12012-12032. doi: 10.1021/acs.jmedchem.4c00721. Epub 2024 Jul 11. J Med Chem. 2024. PMID: 38991154 Free PMC article.
-
Small Molecule Myeloperoxidase (MPO) Inhibition Prevents Delayed Cerebral Injury (DCI) After Subarachnoid Hemorrhage (SAH) in a Murine Model.Neurocrit Care. 2025 Jun;42(3):945-952. doi: 10.1007/s12028-024-02169-x. Epub 2024 Dec 10. Neurocrit Care. 2025. PMID: 39653978 Free PMC article.
-
Myeloperoxidase Inhibitory and Antioxidant Activities of (E)-2-Hydroxy-α-aminocinnamic Acids Obtained through Microwave-Assisted Synthesis.Pharmaceuticals (Basel). 2021 May 27;14(6):513. doi: 10.3390/ph14060513. Pharmaceuticals (Basel). 2021. PMID: 34071735 Free PMC article.
References
-
- Klebanoff S. J. Science. 1970;169:1095. - PubMed
-
- Huang Y., DiDonato J. A., Levison B. S., Schmitt D., Li L., Wu Y., Buffa J., Kim T., Gerstenecker G. S., Gu X., Kadiyala C. S., Wang Z., Culley M. K., Hazen J. E., Didonato A. J., Fu X., Berisha S. Z., Peng D., Nguyen T. T., Liang S., Chuang C. C., Cho L., Plow E. F., Fox P. L., Gogonea V., Tang W. H., Parks J. S., Fisher E. A., Smith J. D., Hazen S. L. Nat. Med. 2014;20:193. - PMC - PubMed
-
- Ikitimur B., Karadag B. Future Cardiol. 2010;6:693. - PubMed
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials

