Alkyne-azide cycloaddition analogues of dehydrozingerone as potential anti-prostate cancer inhibitors via the PI3K/Akt/NF-kB pathway
- PMID: 30108729
- PMCID: PMC6072283
- DOI: 10.1039/c7md00267j
Alkyne-azide cycloaddition analogues of dehydrozingerone as potential anti-prostate cancer inhibitors via the PI3K/Akt/NF-kB pathway
Abstract
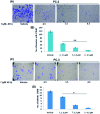
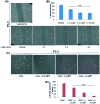
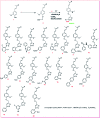
Herein, we report the isolation and synthetic modification of dehydrozingerone (DHZ, 1), a secondary metabolite present in the rhizome of Zingiber officinale. We synthesized O-propargylated dehydrozingerone, which was subsequently coupled by alkyne-azide cycloaddition (3-20) using click chemistry. The compounds (1-20) were evaluated for their in vitro cytotoxic activity in a panel of three cancer cell lines. Among all the DHZ derivatives, 3, 6, 7, 8, 9 and 15 displayed potent cytotoxic potential with an IC50 value ranging from 1.8-3.0 μM in MCF-7, PC-3 and HCT-116 cell lines. Furthermore, compound 7 has proven to be the most potent cytotoxic compound in all the three distinct cancer cell lines and also demonstrated significant anti-invasive potential in prostate cancer. The mechanistic study of compound 7 showed that it not only suppressed the AKT/mTOR signalling which regulates nuclear transcription factor-NF-kB but also augmented the expression of anti-invasive markers E-cadherin and TIMP. Compound 7 significantly decreased the expression of pro-invasive markers vimentin, MMP-2 and MMP-9, respectively. This study underscores an efficient synthetic approach employed to evaluate the structure-activity relationship of dehydrozingerone (1) in search of potential new anticancer agents.
Figures




Similar articles
-
Synthetic modification of hydroxychavicol by Mannich reaction and alkyne-azide cycloaddition derivatives depicting cytotoxic potential.Eur J Med Chem. 2015 Mar 6;92:236-45. doi: 10.1016/j.ejmech.2014.12.047. Epub 2014 Dec 31. Eur J Med Chem. 2015. PMID: 25559204
-
Design, synthesis, and anticancer activity of novel berberine derivatives prepared via CuAAC "click" chemistry as potential anticancer agents.Drug Des Devel Ther. 2014 Aug 6;8:1047-59. doi: 10.2147/DDDT.S63228. eCollection 2014. Drug Des Devel Ther. 2014. PMID: 25120353 Free PMC article.
-
Synthesis and anticancer activity of dimeric podophyllotoxin derivatives.Drug Des Devel Ther. 2018 Oct 9;12:3393-3406. doi: 10.2147/DDDT.S167382. eCollection 2018. Drug Des Devel Ther. 2018. PMID: 30349193 Free PMC article.
-
Discovery of novel anti-HIV agents via Cu(I)-catalyzed azide-alkyne cycloaddition (CuAAC) click chemistry-based approach.Expert Opin Drug Discov. 2016 Sep;11(9):857-71. doi: 10.1080/17460441.2016.1210125. Epub 2016 Jul 21. Expert Opin Drug Discov. 2016. PMID: 27400283 Review.
-
Targeting the interplay between MMP-2, CA II and VEGFR-2 via new sulfonamide-tethered isomeric triazole hybrids; Microwave-assisted synthesis, computational studies and evaluation.Bioorg Chem. 2022 Jul;124:105816. doi: 10.1016/j.bioorg.2022.105816. Epub 2022 Apr 16. Bioorg Chem. 2022. PMID: 35489270 Review.
Cited by
-
miR-601 inhibits proliferation, migration and invasion of prostate cancer stem cells by targeting KRT5 to inactivate the Wnt signaling pathway.Int J Clin Exp Pathol. 2019 Dec 1;12(12):4361-4379. eCollection 2019. Int J Clin Exp Pathol. 2019. PMID: 31933840 Free PMC article.
-
RRBP1 is highly expressed in prostate cancer and correlates with prognosis.Cancer Manag Res. 2019 Apr 23;11:3021-3027. doi: 10.2147/CMAR.S186632. eCollection 2019. Cancer Manag Res. 2019. PMID: 31118771 Free PMC article.
-
Phytochemicals in Prostate Cancer: From Bioactive Molecules to Upcoming Therapeutic Agents.Nutrients. 2019 Jun 29;11(7):1483. doi: 10.3390/nu11071483. Nutrients. 2019. PMID: 31261861 Free PMC article. Review.
-
Effects of orlistat combined with enzalutamide and castration through inhibition of fatty acid synthase in a PC3 tumor-bearing mouse model.Biosci Rep. 2021 May 28;41(5):BSR20204203. doi: 10.1042/BSR20204203. Biosci Rep. 2021. PMID: 33974005 Free PMC article.
-
Molecular mechanism of inhibitory effects of melatonin on prostate cancer cell proliferation, migration and invasion.PLoS One. 2022 Jan 21;17(1):e0261341. doi: 10.1371/journal.pone.0261341. eCollection 2022. PLoS One. 2022. PMID: 35061708 Free PMC article.
References
-
- de Bernardi M., Vidari G., Vita-Finzi P. Phytochemistry. 1976;15(11):1785–1786.
-
- Elias G., Rao M. N. A. Eur. J. Med. Chem. 1988;23(4):379–380.
-
- Wang Y.-J., Pan M.-H., Cheng A.-L., Lin L.-I., Ho Y.-S., Hsieh C.-Y., Lin J.-K. J. Pharm. Biomed. Anal. 1997;15(12):1867–1876. - PubMed
-
- Hampannavar G. A., Karpoormath R., Palkar M. B., Shaikh M. S. Bioorg. Med. Chem. 2016;24(4):501–520. - PubMed
-
- Rajakumar D. V., Rao M. N. A. Mol. Cell. Biochem. 1994;140(1):73–79. - PubMed
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous

