Synthesis and evaluation of small molecules bearing a benzyloxy substituent as novel and potent monoamine oxidase inhibitors
- PMID: 30108765
- PMCID: PMC6072412
- DOI: 10.1039/c6md00586a
Synthesis and evaluation of small molecules bearing a benzyloxy substituent as novel and potent monoamine oxidase inhibitors
Abstract
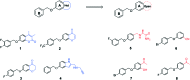
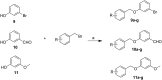
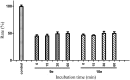
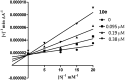
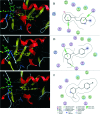
A new series of small molecules bearing a benzyloxy substituent have been designed, synthesized and evaluated for hMAO inhibitory activity in vitro. Most of the compounds were potent and selective MAO-B inhibitors, and were weak inhibitors of MAO-A. In particular, compounds 9e (IC50 = 0.35 μM) and 10e (IC50 = 0.19 μM) were the most potent MAO-B inhibitors, and exhibited the highest selectivity for MAO-B (9e, SI > 285.7-fold and 10e, SI = 146.8-fold). In addition, the structure-activity relationships for MAO-B inhibition indicated that electron-withdrawing groups in the open small molecules were more suitable for MAO-B inhibition, and substitutions at the benzyloxy of the open small molecules, particularly with the halogen substituted benzyloxy, were more favorable for MAO-B inhibition. Molecular docking studies have been done to explain the potent MAO-B inhibition of the open small molecules. Furthermore, the representative compounds 9e and 10e showed low neurotoxicity in SH-SY5Y cells in vitro. So the small molecules bearing the benzyloxy substituent could be used to develop promising drug candidates for the therapy of neurodegenerative diseases.
Figures


References
LinkOut - more resources
Full Text Sources
Other Literature Sources

