New tricks for human farnesyltransferase inhibitor: cancer and beyond
- PMID: 30108801
- PMCID: PMC6072492
- DOI: 10.1039/c7md00030h
New tricks for human farnesyltransferase inhibitor: cancer and beyond
Abstract
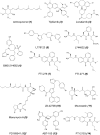
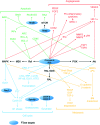
Human protein farnesyltransferase (FTase) catalyzes the addition of a C15-farnesyl lipid group to the cysteine residue located in the COOH-terminal tetrapeptide motif of a variety of important substrate proteins, including well-known Ras protein superfamily. The farnesylation of Ras protein is required both for its normal physiological function, and for the transforming capacity of its oncogenic mutants. Over the last several decades, FTase inhibitors (FTIs) were developed to disrupt the farnesylation of oncogenic Ras as anti-cancer agents, and some of them have entered cancer clinical investigation. On the other hand, some substrates of FTase were demonstrated to be related with other human diseases, including Hutchinson-Gilford progeria syndrome, chronic hepatitis D, and cardiovascular diseases. In this review, we summarize the roles of FTase in malignant transformation, proliferation, apoptosis, angiogenesis, and metastasis of tumor cells, and the recently anticancer clinical research advances of FTIs. The therapeutic prospect of FTIs on several other human diseases is also discussed.
Figures



Similar articles
-
The crystal structure of human protein farnesyltransferase reveals the basis for inhibition by CaaX tetrapeptides and their mimetics.Proc Natl Acad Sci U S A. 2001 Nov 6;98(23):12948-53. doi: 10.1073/pnas.241407898. Epub 2001 Oct 30. Proc Natl Acad Sci U S A. 2001. PMID: 11687658 Free PMC article.
-
Farnesyltransferase inhibitors: a detailed chemical view on an elusive biological problem.Curr Med Chem. 2008;15(15):1478-92. doi: 10.2174/092986708784638825. Curr Med Chem. 2008. PMID: 18537624 Review.
-
Farnesyltransferase and geranylgeranyltransferase I inhibitors and cancer therapy: lessons from mechanism and bench-to-bedside translational studies.Oncogene. 2000 Dec 27;19(56):6584-93. doi: 10.1038/sj.onc.1204146. Oncogene. 2000. PMID: 11426643 Review.
-
Combined p21-activated kinase and farnesyltransferase inhibitor treatment exhibits enhanced anti-proliferative activity on melanoma, colon and lung cancer cell lines.Mol Cancer. 2013 Aug 6;12(1):88. doi: 10.1186/1476-4598-12-88. Mol Cancer. 2013. PMID: 23915247 Free PMC article.
-
Farnesyltransferase and geranylgeranyltransferase I inhibitors in cancer therapy: important mechanistic and bench to bedside issues.Expert Opin Investig Drugs. 2000 Dec;9(12):2767-82. doi: 10.1517/13543784.9.12.2767. Expert Opin Investig Drugs. 2000. PMID: 11093352 Review.
Cited by
-
Blocking the Farnesyl Pocket of PDEδ Reduces Rheb-Dependent mTORC1 Activation and Survival of Tsc2-Null Cells.Front Pharmacol. 2022 Jun 23;13:912688. doi: 10.3389/fphar.2022.912688. eCollection 2022. Front Pharmacol. 2022. PMID: 35814251 Free PMC article.
-
[Effects of farnesyltransferase silencing on the migration and invasion of tongue squamous cell carcinoma].Hua Xi Kou Qiang Yi Xue Za Zhi. 2020 Apr 1;38(2):177-184. doi: 10.7518/hxkq.2020.02.012. Hua Xi Kou Qiang Yi Xue Za Zhi. 2020. PMID: 32314892 Free PMC article. Chinese.
-
Non ABL-directed inhibitors as alternative treatment strategies for chronic myeloid leukemia.Mol Cancer. 2018 Feb 19;17(1):56. doi: 10.1186/s12943-018-0805-1. Mol Cancer. 2018. PMID: 29455672 Free PMC article. Review.
-
Progress on Ras/MAPK Signaling Research and Targeting in Blood and Solid Cancers.Cancers (Basel). 2021 Oct 10;13(20):5059. doi: 10.3390/cancers13205059. Cancers (Basel). 2021. PMID: 34680208 Free PMC article. Review.
-
Assessment of Hematological Toxicity of Adjuvant Chemotherapy in the Complex Therapy of Breast Cancer.Asian Pac J Cancer Prev. 2024 Dec 1;25(12):4123-4128. doi: 10.31557/APJCP.2024.25.12.4123. Asian Pac J Cancer Prev. 2024. PMID: 39733400 Free PMC article.
References
Publication types
LinkOut - more resources
Full Text Sources
Other Literature Sources

