Identification of potent cholecystokinin-B receptor antagonists: synthesis, molecular modeling and anti-cancer activity against pancreatic cancer cells
- PMID: 30108868
- PMCID: PMC6071963
- DOI: 10.1039/c7md00171a
Identification of potent cholecystokinin-B receptor antagonists: synthesis, molecular modeling and anti-cancer activity against pancreatic cancer cells
Abstract
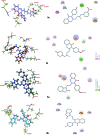
Advanced malignant stages of pancreatic cancer have poor prognosis and very few treatment strategies are available. Pancreatic cancer is known to possess unique growth-related receptors that when activated, stimulate tumour proliferation. Gastrin and its related peptide cholecystokinin (CCK) are also significantly involved in the growth of this cancer type as well as other malignancies through activation of the cholecystokinin-B receptor (CCK-BR). New treatment strategies with CCK-BR antagonists are being suggested that suppress the growth promoting effects of gastrin. In this paper, we report the development of two series of quinazolinone derivatives incorporating hydrazinecarbothioamide (compounds 3a-g) and the hydrazino group (compounds 4a-e) as linkers for developing CCK-BR antagonists. The affinities of the compounds were determined using docking into the CCK-BR homology modeled structure. The compounds were tested for in vitro CCK-BR binding and gastric acid secretion in an isolated lumen-perfused mouse stomach assay. The compounds exhibited CCK-BR binding activity (IC50) in the range of 0.2-975 nM and showed good gastric acid secretion inhibitory activity. Molecular modeling of the compounds was done and pharmacophore mapping results showed good prediction of in vitro activity which correlated well with the experimental antagonistic activity. The compounds were further tested for their cytotoxicity on CCK-BR expressing pancreatic cancer cells. The results of the study provided two potent CCK-BR antagonists which also possess good to moderate growth inhibitory activities against pancreatic cancer cells.
Figures



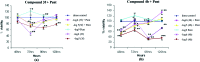
Similar articles
-
Differential expression of gastrin, cholecystokinin-A and cholecystokinin-B receptor mRNA in human pancreatic cancer cell lines.Scand J Gastroenterol. 2001 Jul;36(7):738-43. doi: 10.1080/003655201300192003. Scand J Gastroenterol. 2001. PMID: 11444473
-
Downregulation of the CCK-B receptor in pancreatic cancer cells blocks proliferation and promotes apoptosis.Am J Physiol Gastrointest Liver Physiol. 2012 Jun 1;302(11):G1244-52. doi: 10.1152/ajpgi.00460.2011. Epub 2012 Mar 22. Am J Physiol Gastrointest Liver Physiol. 2012. PMID: 22442157 Free PMC article.
-
Cholecystokinin (CCK) regulates somatostatin secretion through both the CCK-A and CCK-B/gastrin receptors in sheep.J Physiol. 1997 Dec 15;505 ( Pt 3)(Pt 3):811-21. doi: 10.1111/j.1469-7793.1997.811ba.x. J Physiol. 1997. PMID: 9457654 Free PMC article.
-
The Role of Gastrin and CCK Receptors in Pancreatic Cancer and other Malignancies.Int J Biol Sci. 2016 Jan 28;12(3):283-91. doi: 10.7150/ijbs.14952. eCollection 2016. Int J Biol Sci. 2016. PMID: 26929735 Free PMC article. Review.
-
The role of gastrin and cholecystokinin in normal and neoplastic gastrointestinal growth.J Gastroenterol Hepatol. 1995 Mar-Apr;10(2):215-32. doi: 10.1111/j.1440-1746.1995.tb01083.x. J Gastroenterol Hepatol. 1995. PMID: 7787172 Review.
Cited by
-
Design and synthesis of novel quinazolinone-based derivatives as EGFR inhibitors with antitumor activity.J Enzyme Inhib Med Chem. 2022 Dec;37(1):2644-2659. doi: 10.1080/14756366.2022.2118735. J Enzyme Inhib Med Chem. 2022. PMID: 36146940 Free PMC article.
-
Theoretical calculations of molecular descriptors for anticancer activities of 1, 2, 3-triazole-pyrimidine derivatives against gastric cancer cell line (MGC-803): DFT, QSAR and docking approaches.Heliyon. 2020 May 21;6(5):e03926. doi: 10.1016/j.heliyon.2020.e03926. eCollection 2020 May. Heliyon. 2020. PMID: 32462084 Free PMC article.
References
-
- Hidalgo M. N. Engl. J. Med. 2010;362:1605–1617. - PubMed
-
- Siegel R., Ma J., Zou Z., Jemal A. Ca-Cancer J. Clin. 2014;64:9–29. - PubMed
-
- Ryan D. P., Hong T. S., Bardeesy N. N. Engl. J. Med. 2014;371:1039–1049. - PubMed
-
- Rahib L., Smith B. D., Aizenberg R., Rosenzweig A. B., Fleshman J. M., Matrisian L. M. Cancer Res. 2014;74:2913–2921. - PubMed
-
- Smith J. P., Rickabaugh C. A., McLaughlin P. J., Zagon I. S. Am. J. Physiol. 1993;265:G149–155. - PubMed
LinkOut - more resources
Full Text Sources
Other Literature Sources

