Recent advances in combretastatin based derivatives and prodrugs as antimitotic agents
- PMID: 30108870
- PMCID: PMC6072414
- DOI: 10.1039/c7md00227k
Recent advances in combretastatin based derivatives and prodrugs as antimitotic agents
Abstract
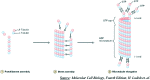
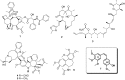
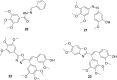
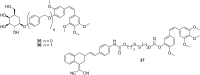
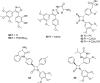
The dynamic and crucial role of tubulin in different cellular functions rendered it a promising target in anticancer drug development. Combretastatin A-4 (CA-4), an inhibitor of tubulin polymerization isolated from natural sources, is a lead molecule with significant cytotoxicity against tumour cells. Owing to its non polar nature it exhibits low solubility in natural biological fluids, thereby prompting the development of new CA-4 based derivatives. The modification of this lead molecule was mostly carried out by keeping the crucial cis-orientation of the double bond intact, along with a trimethoxyphenyl aromatic ring, by employing different approaches. The issue of solubility was also addressed by the development of water soluble prodrugs of CA-4. The present review highlights the investigations into the parallel development of both new CA-4 based derivatives and prodrugs in the past few years.
Figures




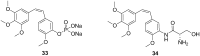





Similar articles
-
Combretastatin-based compounds with therapeutic characteristics: a patent review.Expert Opin Ther Pat. 2019 Sep;29(9):703-731. doi: 10.1080/13543776.2019.1651841. Epub 2019 Aug 8. Expert Opin Ther Pat. 2019. PMID: 31369715 Review.
-
Design, synthesis, and biological evaluation of water-soluble amino acid prodrug conjugates derived from combretastatin, dihydronaphthalene, and benzosuberene-based parent vascular disrupting agents.Bioorg Med Chem. 2016 Mar 1;24(5):938-956. doi: 10.1016/j.bmc.2016.01.007. Epub 2016 Jan 6. Bioorg Med Chem. 2016. PMID: 26852340 Free PMC article.
-
Interactions of tubulin with potent natural and synthetic analogs of the antimitotic agent combretastatin: a structure-activity study.Mol Pharmacol. 1988 Aug;34(2):200-8. Mol Pharmacol. 1988. PMID: 3412321
-
Combretastatin A-4 analogues as antimitotic antitumor agents.Curr Med Chem. 2003 Sep;10(17):1697-722. doi: 10.2174/0929867033457151. Curr Med Chem. 2003. PMID: 12871118 Review.
-
Developments of combretastatin A-4 derivatives as anticancer agents.Curr Med Chem. 2011;18(4):523-38. doi: 10.2174/092986711794480221. Curr Med Chem. 2011. PMID: 21143124 Review.
Cited by
-
Novel Indole-Tethered Chromene Derivatives: Synthesis, Cytotoxic Properties, and Key Computational Insights.Pharmaceuticals (Basel). 2023 Feb 22;16(3):333. doi: 10.3390/ph16030333. Pharmaceuticals (Basel). 2023. PMID: 36986433 Free PMC article.
-
Target-based anticancer indole derivatives and insight into structure‒activity relationship: A mechanistic review update (2018-2021).Acta Pharm Sin B. 2022 Jul;12(7):3006-3027. doi: 10.1016/j.apsb.2022.03.021. Epub 2022 Apr 1. Acta Pharm Sin B. 2022. PMID: 35865090 Free PMC article. Review.
-
Imidazole Analogs of Vascular-Disrupting Combretastatin A-4 with Pleiotropic Efficacy against Resistant Colorectal Cancer Models.Int J Mol Sci. 2021 Dec 3;22(23):13082. doi: 10.3390/ijms222313082. Int J Mol Sci. 2021. PMID: 34884888 Free PMC article.
-
Microtubule Destabilizing Sulfonamides as an Alternative to Taxane-Based Chemotherapy.Int J Mol Sci. 2021 Feb 14;22(4):1907. doi: 10.3390/ijms22041907. Int J Mol Sci. 2021. PMID: 33673002 Free PMC article.
-
Biological aspects in controlling angiogenesis: current progress.Cell Mol Life Sci. 2022 Jun 7;79(7):349. doi: 10.1007/s00018-022-04348-5. Cell Mol Life Sci. 2022. PMID: 35672585 Free PMC article. Review.
References
-
- Mitchison T. J. Annu. Rev. Cell Biol. 1988;4:527–549. - PubMed
-
- Amos L. A., in eLS, John Wiley & Sons, Ltd, 2001.
-
- Waterman-Storer C. M., Salmon E. D. Curr. Biol. 1997;7:R369–R372. - PubMed
-
- Jordan M. A., Wilson L. Nat. Rev. Cancer. 2004;4:253–265. - PubMed
-
- Altmann K. H. Curr. Opin. Chem. Biol. 2001;5:424–431. - PubMed
Publication types
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

