Click chemistry-assisted synthesis of triazolo linked podophyllotoxin conjugates as tubulin polymerization inhibitors
- PMID: 30108892
- PMCID: PMC6084182
- DOI: 10.1039/c7md00273d
Click chemistry-assisted synthesis of triazolo linked podophyllotoxin conjugates as tubulin polymerization inhibitors
Abstract
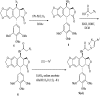
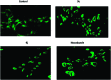
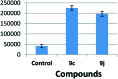
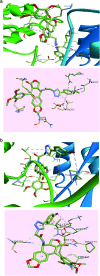
A series of new triazolo linked 4β-amidopodophyllotoxin conjugates (9a-l) were synthesized using click chemistry and evaluated for their antitumor activity against four human cancer cell lines. Among them, two compounds (9c and 9j) showed significant anticancer activity with IC50 values of 0.9 and 0.07 μM, respectively. Biological studies are conducted into the cell-cycle distribution of these conjugates inducing G2/M-phase arrest, apart from an increase in the levels of caspase-3 proteins, followed by apoptotic cell death. A tubulin polymerization assay analysis showed that these compounds effectively inhibit microtubule assembly in HeLa cells and, moreover, Hoechst 33258 and Immunohistochemistry staining suggest that these compounds induce cell death by apoptosis. The docking studies showed that compounds 9c and 9j interact and bind efficiently with the tubulin protein at the colchicine site.
Figures





References
-
- Slevin L. M. L. Cancer. 1991;67:319–329. - PubMed
-
- Hande K. R. Eur. J. Cancer. 1998;34:1514–1521. - PubMed
-
- Caner B. A. and Longo D. L., Cancer therapy and biotherapy, Lippincott-Raven Publishers, New York, 1996.
-
- Yuan P., Xu B. H., Wang J. Y., Ma F., Fan Y., Li Q., Zhang P. J. Clin. Med. 2012;125:79–139.
-
- Burden D. A., Osheroff N. Biochim. Biophys. Acta, Gen. Subj. 1998;1400:139–154. - PubMed
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials

