Sugar modified pyrimido[4,5- b]indole nucleosides: synthesis and antiviral activity
- PMID: 30108897
- PMCID: PMC6084004
- DOI: 10.1039/c7md00319f
Sugar modified pyrimido[4,5- b]indole nucleosides: synthesis and antiviral activity
Abstract
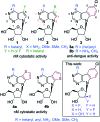
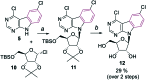
Three types of sugar modified pyrimido[4,5-b]indole nucleosides (2'-deoxy-2'-fluororibo-, 2'-deoxy-2'-fluoroarabino- and arabinonucleosides) were synthesized by glycosylation of 4,6-dichloropyrimido[4,5-b]indole followed by modification of sugar moiety and introduction of substituents into position 4 by cross-coupling reactions or nucleophilic substitutions. Some 2'-fluororibo- and 2'-fluoroarabinonucleosides displayed interesting anti-HCV activities (IC50 = 1.6-20 μM) and the latter compounds also some anti-dengue activities (IC50 = 10.8-40 μM).
Figures




Similar articles
-
Sugar-modified derivatives of cytostatic 7-(het)aryl-7-deazaadenosines: 2'-C-methylribonucleosides, 2'-deoxy-2'-fluoroarabinonucleosides, arabinonucleosides and 2'-deoxyribonucleosides.Bioorg Med Chem. 2012 Sep 1;20(17):5202-14. doi: 10.1016/j.bmc.2012.07.003. Epub 2012 Jul 20. Bioorg Med Chem. 2012. PMID: 22877872
-
Synthesis and antiviral activity of 4,6-disubstituted pyrimido[4,5-b]indole ribonucleosides.Bioorg Med Chem. 2012 Oct 15;20(20):6123-33. doi: 10.1016/j.bmc.2012.08.021. Epub 2012 Aug 24. Bioorg Med Chem. 2012. PMID: 22985963
-
Design, synthesis, and antiviral activity of certain 3-substituted 2,5,6-trichloroindole nucleosides.J Med Chem. 2004 Nov 4;47(23):5753-65. doi: 10.1021/jm0400146. J Med Chem. 2004. PMID: 15509174
-
Sugar-modified nucleosides in past 10 years, a review.Curr Med Chem. 2001 Mar;8(4):385-423. doi: 10.2174/0929867013373471. Curr Med Chem. 2001. PMID: 11172696 Review.
-
Glycosylation reactions mediated by hypervalent iodine: application to the synthesis of nucleosides and carbohydrates.Beilstein J Org Chem. 2018 Jun 28;14:1595-1618. doi: 10.3762/bjoc.14.137. eCollection 2018. Beilstein J Org Chem. 2018. PMID: 30013687 Free PMC article. Review.
Cited by
-
In silico DFT study, molecular docking, and ADMET predictions of cytidine analogs with antimicrobial and anticancer properties.In Silico Pharmacol. 2021 Jul 6;9(1):42. doi: 10.1007/s40203-021-00102-0. eCollection 2021. In Silico Pharmacol. 2021. PMID: 34295612 Free PMC article.
-
An insight into the recent developments in anti-infective potential of indole and associated hybrids.J Mol Struct. 2022 Aug 5;1261:132808. doi: 10.1016/j.molstruc.2022.132808. Epub 2022 Mar 11. J Mol Struct. 2022. PMID: 35291692 Free PMC article. Review.
-
Enzymatic synthesis of nucleoside analogues from uridines and vinyl esters in a continuous-flow microreactor.RSC Adv. 2018 Apr 3;8(23):12614-12618. doi: 10.1039/c8ra01030g. eCollection 2018 Apr 3. RSC Adv. 2018. PMID: 35541271 Free PMC article.
-
Indole - a promising pharmacophore in recent antiviral drug discovery.RSC Med Chem. 2020 Nov 6;11(12):1335-1353. doi: 10.1039/d0md00288g. eCollection 2020 Dec 17. RSC Med Chem. 2020. PMID: 34095843 Free PMC article. Review.
-
Triterpenoid-PEG Ribbons Targeting Selectivity in Pharmacological Effects.Biomedicines. 2021 Aug 3;9(8):951. doi: 10.3390/biomedicines9080951. Biomedicines. 2021. PMID: 34440155 Free PMC article.
References
-
- Jordheim L. P., Durantel D., Zoulim F., Dumontet C. Nat. Rev. Drug Discovery. 2013;12:447–464. - PubMed
-
- Sofia M. J., Bao D., Chang W., Du J., Nagarathnam D., Rachakonda S., Reddy P. G., Ross B. S., Wang P., Zhang H.-R., Bansal S., Espiritu C., Keilman M., Lam A. M., Steuer H. M. M., Niu C., Otto M. J., Furman P. A. J. Med. Chem. 2010;53:7202–7218. - PubMed
-
- Siegel D., Hui H. C., Doerffler E., Clarke M. O., Chun K., Zhang L., Neville S., Carra E., Lew W., Ross B., Wang Q., Wolfe L., Jordan R., Soloveva V., Knox J., Perry J., Perron M., Stray K. M., Barauskas O., Feng J. Y., Xu Y., Lee G., Rheingold A. L., Ray A. S., Bannister R., Strickley R., Swaminathan S., Lee W. A., Bavari S., Cihlar T., Lo M. K., Warren T. K., Mackman R. L. J. Med. Chem. 2017;60:1648–1661. - PubMed
LinkOut - more resources
Full Text Sources
Other Literature Sources

