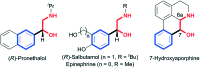
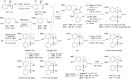
Discovery of 7-hydroxyaporphines as conformationally restricted ligands for beta-1 and beta-2 adrenergic receptors
- PMID: 30108929
- PMCID: PMC6083790
- DOI: 10.1039/c7md00656j
Discovery of 7-hydroxyaporphines as conformationally restricted ligands for beta-1 and beta-2 adrenergic receptors
Abstract
A series of (-)-nornuciferidine derivatives was synthesized and the non-natural enantiomer of the aporphine alkaloid was discovered to be a potent β1- and β2-adrenergic receptor ligand that antagonized isoproterenol and procaterol induced cyclic AMP increases from adenylyl cyclase, respectively. Progressive deconstruction of the tetracyclic scaffold to less complex cyclic and acyclic analogues revealed that the conformationally restricted (6a-R,7-R)-7-hydroxyaporphine 2 (AK-2-202) was necessary for efficient receptor binding and antagonism.
Figures
Similar articles
-
Differential coupling to positive inotropic responses of cyclic AMP produced by stimulation of beta 1- and beta 2-adrenergic receptors.J Cardiovasc Pharmacol. 1989 Jan;13(1):64-75. J Cardiovasc Pharmacol. 1989. PMID: 2468937
-
Functional beta1- and beta2-adrenoceptors in the left and right atrium of pre-hypertensive rats.J Pharm Pharmacol. 2002 Oct;54(10):1407-12. doi: 10.1211/002235702760345509. J Pharm Pharmacol. 2002. PMID: 12396304
-
Beta2 adrenergic receptors mediate cAMP, tissue-type plasminogen activator and transferrin production in rat Sertoli cells.Mol Cell Endocrinol. 1998 Jul 25;142(1-2):75-86. doi: 10.1016/s0303-7207(98)00115-4. Mol Cell Endocrinol. 1998. PMID: 9783905
-
Regulation of phospholamban and troponin-I phosphorylation in the intact rat cardiomyocytes by adrenergic and cholinergic stimuli: roles of cyclic nucleotides, calcium, protein kinases and phosphatases and depolarization.Mol Cell Biochem. 1995 Aug-Sep;149-150:103-26. doi: 10.1007/BF01076569. Mol Cell Biochem. 1995. PMID: 8569720 Review.
-
Beta-adrenergic receptors, cyclic AMP, and ion transport in the avian erythrocyte.Adv Cyclic Nucleotide Res. 1975;5:117-32. Adv Cyclic Nucleotide Res. 1975. PMID: 165661 Review.
Cited by
-
Synthesis of the Tetracyclic Framework of Polycyclic Spiro Lignan Natural Products.ACS Omega. 2020 Apr 8;5(15):9007-9012. doi: 10.1021/acsomega.0c00976. eCollection 2020 Apr 21. ACS Omega. 2020. PMID: 32337465 Free PMC article.
References
LinkOut - more resources
Full Text Sources
Other Literature Sources