Conformationally restricted benzothienoazepine respiratory syncytial virus inhibitors: their synthesis, structural analysis and biological activities
- PMID: 30108949
- PMCID: PMC6072213
- DOI: 10.1039/c8md00033f
Conformationally restricted benzothienoazepine respiratory syncytial virus inhibitors: their synthesis, structural analysis and biological activities
Erratum in
-
Correction: Conformationally restricted benzothienoazepine respiratory syncytial virus inhibitors: their synthesis, structural analysis and biological activities.Medchemcomm. 2018 Mar 26;9(4):746. doi: 10.1039/c8md90016g. eCollection 2018 Apr 1. Medchemcomm. 2018. PMID: 30288214 Free PMC article.
Abstract
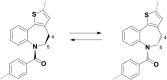
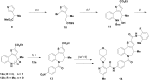
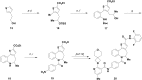
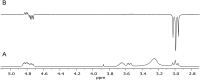
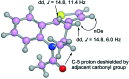
Atropisomeric drug substances are known to have different biological properties. Compounds containing the N-benzoylbenzazepine motif have been shown to exhibit energetically restricted rotation around the Ar(CO)N axis. Herein we report, for the first time, the synthesis, physical characterisation and anti-viral profiles of a series of C-4 and C-5 methylated thieno-benzazepines. NMR analysis reveals that incorporation of a single additional substituent at either of these loci influences the conformational dynamics of the azepine ring system. In the case of the C-5 alkyl analogues, the influence of the new stereocentre is so pronounced that its absolute configuration determines which unique atropisomer is obtained following the generation of the benzazepine nucleus. Screening of the alkylated derivatives for their anti-respiratory syncytial virus (RSV) activity indicates that the desired viral pathogenicity is strongly associated with the conformation adopted by the modified tricyclic scaffolds. This is particularly evident in the case of the C-5 homologues in which one atropisomer was found to be potently active and the other essentially inert. These results provide compelling evidence that we have determined the bioactive conformation shared by RSV inhibitors that employ the thienobenazapine nucleus as their core molecular architecture. Furthermore, the understanding obtained from these studies may make it possible to design improved agents against RSV infection in the future.
Figures
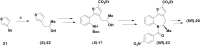
Similar articles
-
Discovery of novel benzothienoazepine derivatives as potent inhibitors of respiratory syncytial virus.Bioorg Med Chem Lett. 2017 May 15;27(10):2201-2206. doi: 10.1016/j.bmcl.2017.03.053. Epub 2017 Mar 23. Bioorg Med Chem Lett. 2017. PMID: 28372911
-
Atropisomerism in Drug Discovery: A Medicinal Chemistry Perspective Inspired by Atropisomeric Class I PI3K Inhibitors.Acc Chem Res. 2022 Sep 20;55(18):2581-2593. doi: 10.1021/acs.accounts.2c00485. Epub 2022 Sep 7. Acc Chem Res. 2022. PMID: 36069734
-
Correction: Conformationally restricted benzothienoazepine respiratory syncytial virus inhibitors: their synthesis, structural analysis and biological activities.Medchemcomm. 2018 Mar 26;9(4):746. doi: 10.1039/c8md90016g. eCollection 2018 Apr 1. Medchemcomm. 2018. PMID: 30288214 Free PMC article.
-
Respiratory syncytial virus (RSV) evades the human adaptive immune system by skewing the Th1/Th2 cytokine balance toward increased levels of Th2 cytokines and IgE, markers of allergy--a review.Virus Genes. 2006 Oct;33(2):235-52. doi: 10.1007/s11262-006-0064-x. Virus Genes. 2006. PMID: 16972040 Review.
-
Relationship between respiratory syncytial virus bronchiolitis and future obstructive airway diseases.Eur Respir J. 2001 Dec;18(6):1044-58. doi: 10.1183/09031936.01.00254101. Eur Respir J. 2001. PMID: 11829086 Review.
Cited by
-
Troponoid Atropisomerism: Studies on the Configurational Stability of Tropone-Amide Chiral Axes.Org Lett. 2019 Apr 5;21(7):2412-2415. doi: 10.1021/acs.orglett.9b00707. Epub 2019 Mar 14. Org Lett. 2019. PMID: 30869521 Free PMC article.
-
Substituted N-(4-amino-2-chlorophenyl)-5-chloro-2-hydroxybenzamide analogues potently inhibit respiratory syncytial virus (RSV) replication and RSV infection-associated inflammatory responses.Bioorg Med Chem. 2021 Jun 1;39:116157. doi: 10.1016/j.bmc.2021.116157. Epub 2021 Apr 18. Bioorg Med Chem. 2021. PMID: 33895704 Free PMC article.
References
-
- Clayden J., Moran W. J., Edwards P. J., LaPlante S. R. Angew. Chem., Int. Ed. 2009;48:6398–6401. - PubMed
- LaPlante S. R., Fader L. D., Fandrick K. R., Fandrick D. R., Hucke O., Kemper R., Miller S. P. F., Edwards P. J. J. Med. Chem. 2011;54:7005–7022. - PubMed
- Glunz P. W. Bioorg. Med. Chem. Lett. 2018;28:53–60. - PubMed
-
- Oki M. Top. Stereochem. 1983;14:1–76.
-
- Fukuyama Y., Asakawa Y. J. Chem. Soc., Perkin Trans. 1. 1991;0:2737–2741.
-
- Ito C., Thoyama Y., Omura M., Kajiura I., Furukawa H. Chem. Pharm. Bull. 1993;41:2096–2100.
- Bringmann G., Tasler S., Endress H., Kraus J., Messer K., Wohlfarth M., Lobin W. J. Am. Chem. Soc. 2001;123:2703–2711. - PubMed
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous

