Peptidomics of potato protein hydrolysates: implications of post-translational modifications in food peptide structure and behaviour
- PMID: 30109062
- PMCID: PMC6083715
- DOI: 10.1098/rsos.172425
Peptidomics of potato protein hydrolysates: implications of post-translational modifications in food peptide structure and behaviour
Abstract
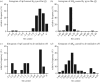
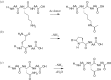
Post-translational modifications (PTMs) often occur in proteins and play a regulatory role in protein function. There is an increasing interest in the bioactivity of food protein-derived peptides, but the occurrence of PTMs and their influence on food peptide structure and behaviour remain largely unknown. In this study, the shotgun-based peptidomics strategy was used to identify the occurrence of PTMs in peptides generated from potato protein hydrolysis using digestive proteases. Diverse PTMs were found in the potato peptides, including acetylation of lysine, N-terminal of proteins and peptides, C-terminal amidation, de-amidation of asparagine/glutamine, methylation and trimethylation, methionine oxidation and N-terminal pyro-glutamyl residue formation. The modifications may have been formed naturally or as a result of chemical reactions during isolation and enzymatic processing of the potato proteins. Most of the PTMs were calculated to decrease the isoelectric point and increase molecular hydrophobicity of the peptides, which will influence their bioactivity while also potentially altering their solubility in an aqueous environment. This is the first study to unravel that food-derived peptides can be widely modified by PTMs associated with notable changes in peptide chemical properties. The findings have broader implications on the bioavailability, biomolecular interactions and biological activities of food peptides.
Keywords: bioactive peptides; food proteins; hydrophobicity; isoelectric point; post-translational modification.
Conflict of interest statement
We declare we have no competing interests.
Figures
References
-
- Nongonierma AB, FitzGerald RJ. 2016. Learnings from quantitative structure-activity relationship (QSAR) studies with respect to food protein-derived bioactive peptides: a review. RSC Adv. 6, 75 400–75 413. ( 10.1039/C6RA12738J) - DOI
-
- Pripp AH, Isaksson T, Stepaniak L, Rhaug TS. 2004. Quantitative structure-activity relationship modelling of ACE-inhibitory peptides derived from milk proteins. Eur. Food Res. Technol. 219, 579–583. ( 10.1007/s00217-004-1004-4) - DOI
Associated data
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous
