Olmutinib (HM61713) reversed multidrug resistance by inhibiting the activity of ATP-binding cassette subfamily G member 2 in vitro and in vivo
- PMID: 30109181
- PMCID: PMC6089862
- DOI: 10.1016/j.apsb.2018.06.002
Olmutinib (HM61713) reversed multidrug resistance by inhibiting the activity of ATP-binding cassette subfamily G member 2 in vitro and in vivo
Abstract
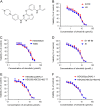
Overexpressing of ATP-binding cassette (ABC) transporters is the essential cause of multidrug resistance (MDR), which is a significant hurdle to the success of chemotherapy in many cancers. Therefore, inhibiting the activity of ABC transporters may be a logical approach to circumvent MDR. Olmutinib is an epidermal growth factor receptor (EGFR) tyrosine kinase inhibitor (TKI), which has been approved in South Korea for advanced EGFR T790M-positive non-small cell lung cancer (NSCLC). Here, we found that olmutinib significantly increased the sensitivity of chemotherapy drug in ABCG2-overexpressing cells. Furthermore, olmutinib could also increase the retention of doxorubicin (DOX) and rhodamine 123 (Rho 123) in ABC transporter subfamily G member 2 (ABCG2)-overexpressing cells. In addition, olmutinib was found to stimulate ATPase activity and inhibit photolabeling of ABCG2 with [125I]-iodoarylazidoprazosin (IAAP). However, olmutinib neither altered ABCG2 expression at protein and mRNA levels nor blocked EGFR, Her-2 downstream signaling of AKT and ERK. Importantly, olmutinib enhanced the efficacy of topotecan on the inhibition of S1-MI-80 cell xenograft growth. All the results suggest that olmutinib reverses ABCG2-mediated MDR by binding to ATP bind site of ABCG2 and increasing intracellular chemotherapeutic drug accumulation. Our findings encouraged to further clinical investigation on combination therapy of olmutinib with conventional chemotherapeutic drugs in ABCG2-overexpressing cancer patients.
Keywords: ABC, adenosine triphosphate (ATP)-binding cassette; ABCG2; ABCG2, ABC transporter subfamily G member 2; ATPase; Chemotherapy; DDP, cisplatin; DMEM, Dulbecco׳s modified Eagle׳s medium; DMSO, dimethyl sulfoxide; DOX, doxorubicin; FTC, fumitremorgin C; IAAP, iodoarylazidoprazosin; MDR, multidrug resistance; MTT, 3-(4,5-dimethylthiazol-2yl)-2,5-diphenyltetrazoliumbromide; MX, methotrexate; Multidrug resistance; Olmutinib; PCR, polymerase chain reaction; Rho 123, rhodamine 123; TKI, tyrosine kinase inhibitor; Tyrosine kinase inhibitor; VRP, verapamil.
Figures






Similar articles
-
Rociletinib (CO-1686) enhanced the efficacy of chemotherapeutic agents in ABCG2-overexpressing cancer cells in vitro and in vivo.Acta Pharm Sin B. 2020 May;10(5):799-811. doi: 10.1016/j.apsb.2020.01.008. Epub 2020 Jan 26. Acta Pharm Sin B. 2020. PMID: 32528828 Free PMC article.
-
Dacomitinib potentiates the efficacy of conventional chemotherapeutic agents via inhibiting the drug efflux function of ABCG2 in vitro and in vivo.J Exp Clin Cancer Res. 2018 Feb 20;37(1):31. doi: 10.1186/s13046-018-0690-x. J Exp Clin Cancer Res. 2018. PMID: 29458405 Free PMC article.
-
Olmutinib (BI1482694/HM61713), a Novel Epidermal Growth Factor Receptor Tyrosine Kinase Inhibitor, Reverses ABCG2-Mediated Multidrug Resistance in Cancer Cells.Front Pharmacol. 2018 Oct 9;9:1097. doi: 10.3389/fphar.2018.01097. eCollection 2018. Front Pharmacol. 2018. PMID: 30356705 Free PMC article.
-
Repositioning of Tyrosine Kinase Inhibitors as Antagonists of ATP-Binding Cassette Transporters in Anticancer Drug Resistance.Cancers (Basel). 2014 Sep 29;6(4):1925-52. doi: 10.3390/cancers6041925. Cancers (Basel). 2014. PMID: 25268163 Free PMC article. Review.
-
The role of ATP-binding cassette transporter A2 in childhood acute lymphoblastic leukemia multidrug resistance.Iran J Ped Hematol Oncol. 2014;4(3):118-26. Epub 2014 Jul 20. Iran J Ped Hematol Oncol. 2014. PMID: 25254091 Free PMC article. Review.
Cited by
-
ERK is a Pivotal Player of Chemo-Immune-Resistance in Cancer.Int J Mol Sci. 2019 May 21;20(10):2505. doi: 10.3390/ijms20102505. Int J Mol Sci. 2019. PMID: 31117237 Free PMC article. Review.
-
A Danshensu-Tetramethylpyrazine Conjugate DT-010 Overcomes Multidrug Resistance in Human Breast Cancer.Front Pharmacol. 2019 Jun 26;10:722. doi: 10.3389/fphar.2019.00722. eCollection 2019. Front Pharmacol. 2019. PMID: 31293428 Free PMC article.
-
Adagrasib, a KRAS G12C inhibitor, reverses the multidrug resistance mediated by ABCB1 in vitro and in vivo.Cell Commun Signal. 2022 Sep 14;20(1):142. doi: 10.1186/s12964-022-00955-8. Cell Commun Signal. 2022. PMID: 36104708 Free PMC article.
-
Editorial Preface for Targeted Cancer Therapy.Acta Pharm Sin B. 2018 Jul;8(4):501-502. doi: 10.1016/j.apsb.2018.07.003. Epub 2018 Jul 25. Acta Pharm Sin B. 2018. PMID: 30109174 Free PMC article. No abstract available.
-
Mitomycin C enhanced the efficacy of PD-L1 blockade in non-small cell lung cancer.Signal Transduct Target Ther. 2020 Aug 28;5(1):141. doi: 10.1038/s41392-020-0200-4. Signal Transduct Target Ther. 2020. PMID: 32855386 Free PMC article.
References
-
- Gottesman M.M. Mechanisms of cancer drug resistance. Annu Rev Med. 2002;53:615–627. - PubMed
-
- Deeley R.G., Westlake C., Cole S.P. Transmembrane transport of endo- and xenobiotics by mammalian ATP-binding cassette multidrug resistance proteins. Physiol Rev. 2006;86:849–899. - PubMed
-
- Quintieri L., Fantin M., Vizier C. Springer; New York: 2007. Identification of molecular determinants of tumor sensitivity and resistance to anticancer drugs. - PubMed
-
- Lowe S.W., Ruley H.E., Jacks T., Housman D.E. p53-dependent apoptosis modulates the cytotoxicity of anticancer agents. Cell. 1993;74:957–967. - PubMed
-
- Synold T.W., Dussault I., Forman B.M. The orphan nuclear receptor SXR coordinately regulates drug metabolism and efflux. Nat Med. 2001;7:584–590. - PubMed
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous

