Overexpression of microRNA-34a Attenuates Proliferation and Induces Apoptosis in Pituitary Adenoma Cells via SOX7
- PMID: 30109259
- PMCID: PMC6083820
- DOI: 10.1016/j.omto.2018.07.001
Overexpression of microRNA-34a Attenuates Proliferation and Induces Apoptosis in Pituitary Adenoma Cells via SOX7
Abstract
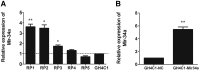
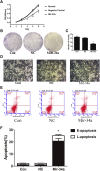
Pituitary adenomas constitute one of the most common intracranial tumors and are typically benign. However, the role of the tumor suppressor microRNA-34a (miR-34a), which is implicated in other cancers, in pituitary adenoma pathogenesis remains largely unknown. miR-34a expression was compared between GH4C1 cancer cells and normal cells derived from the pituitary gland of Rattus norvegicus, and the effects of miR-34a on GH4C1 cell proliferation and apoptosis were examined. miR-34a target genes were identified and analyzed computationally. The mRNA levels of the miR-34a target genes were measured using qRT-PCR, and the protein levels of the differentially expressed targets were assessed by western blotting. miR-34a expression was significantly lower in GH4C1 cells, whereas miR-34a overexpression significantly inhibited GH4C1 cell proliferation and promoted cell apoptosis though SRY-box 7 (SOX7). Our data facilitate the development of a better understanding of the pathogenesis and treatment of pituitary adenomas by elucidating the crucial role of miR-34a in the development of pituitary adenomas.
Keywords: SOX7; apoptosis; cell division; microRNAs; pituitary adenoma.
Figures


Similar articles
-
MicroRNA-34a Attenuates Paclitaxel Resistance in Prostate Cancer Cells via Direct Suppression of JAG1/Notch1 Axis.Cell Physiol Biochem. 2018;50(1):261-276. doi: 10.1159/000494004. Epub 2018 Oct 3. Cell Physiol Biochem. 2018. PMID: 30282072
-
MiR-34a targets GAS1 to promote cell proliferation and inhibit apoptosis in papillary thyroid carcinoma via PI3K/Akt/Bad pathway.Biochem Biophys Res Commun. 2013 Nov 29;441(4):958-63. doi: 10.1016/j.bbrc.2013.11.010. Epub 2013 Nov 9. Biochem Biophys Res Commun. 2013. PMID: 24220341
-
MicroRNA-34a targets Bcl-2 and sensitizes human hepatocellular carcinoma cells to sorafenib treatment.Technol Cancer Res Treat. 2014 Feb;13(1):77-86. doi: 10.7785/tcrt.2012.500364. Epub 2013 Jul 11. Technol Cancer Res Treat. 2014. PMID: 23862748
-
Expanding the Biotherapeutics Realm via miR-34a: "Potent Clever Little" Agent in Breast Cancer Therapy.Curr Pharm Biotechnol. 2019;20(8):665-673. doi: 10.2174/1389201020666190617162042. Curr Pharm Biotechnol. 2019. PMID: 31244419 Review.
-
microRNA-34a as a Therapeutic Agent against Human Cancer.J Clin Med. 2015 Nov 16;4(11):1951-9. doi: 10.3390/jcm4111951. J Clin Med. 2015. PMID: 26580663 Free PMC article. Review.
Cited by
-
Evaluation of MiR-1908-3p as a novel serum biomarker for breast cancer and analysis its oncogenic function and target genes.BMC Cancer. 2020 Jul 10;20(1):644. doi: 10.1186/s12885-020-07125-4. BMC Cancer. 2020. PMID: 32650755 Free PMC article.
-
The Emerging Role of Non-Coding RNAs in Pituitary Gland Tumors and Meningioma.Cancers (Basel). 2021 Nov 28;13(23):5987. doi: 10.3390/cancers13235987. Cancers (Basel). 2021. PMID: 34885097 Free PMC article. Review.
-
miR-489-3p/SIX1 Axis Regulates Melanoma Proliferation and Glycolytic Potential.Mol Ther Oncolytics. 2019 Nov 27;16:30-40. doi: 10.1016/j.omto.2019.11.001. eCollection 2020 Mar 27. Mol Ther Oncolytics. 2019. PMID: 32258386 Free PMC article.
-
Analysis of whole genome-wide microRNA transcriptome profiling in invasive pituitary adenomas and non-invasive pituitary adenomas.Chin Neurosurg J. 2019 Dec 2;5:27. doi: 10.1186/s41016-019-0177-4. eCollection 2019. Chin Neurosurg J. 2019. PMID: 32922926 Free PMC article.
-
[Differences in plasma miRNA levels in inferior petrosal sinus samples of patients with ACTH-dependent Cushing's syndrome].Probl Endokrinol (Mosk). 2021 Nov 12;67(6):18-30. doi: 10.14341/probl12817. Probl Endokrinol (Mosk). 2021. PMID: 35018758 Free PMC article. Russian.
References
-
- Ezzat S., Asa S.L., Couldwell W.T., Barr C.E., Dodge W.E., Vance M.L., McCutcheon I.E. The prevalence of pituitary adenomas: a systematic review. Cancer. 2004;101:613–619. - PubMed
-
- Pichard C., Gerber S., Laloi M., Kujas M., Clemenceau S., Ponvert D., Bruckert E., Turpin G. Pituitary carcinoma: report of an exceptional case and review of the literature. J. Endocrinol. Invest. 2002;25:65–72. - PubMed
-
- Cristina C., Luque G.M., Demarchi G., Lopez Vicchi F., Zubeldia-Brenner L., Perez Millan M.I., Perrone S., Ornstein A.M., Lacau-Mengido I.M., Berner S.I., Becu-Villalobos D. Angiogenesis in pituitary adenomas: human studies and new mutant mouse models. Int. J. Endocrinol. 2014;2014:608497. - PMC - PubMed
-
- Hewedi I.H., Osman W.M., El Mahdy M.M. Differential expression of cyclin D1 in human pituitary tumors: relation to MIB-1 and p27/Kip1 labeling indices. J. Egypt. Natl. Canc. Inst. 2011;23:171–179. - PubMed
LinkOut - more resources
Full Text Sources
Other Literature Sources

