Oxidative Stress, Intrauterine Growth Restriction, and Developmental Programming of Type 2 Diabetes
- PMID: 30109821
- PMCID: PMC6230552
- DOI: 10.1152/physiol.00023.2018
Oxidative Stress, Intrauterine Growth Restriction, and Developmental Programming of Type 2 Diabetes
Abstract
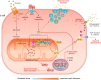
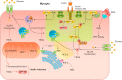
Intrauterine growth restriction (IUGR) leads to reduced birth weight and the development of metabolic diseases such as Type 2 diabetes in adulthood. Mitochondria dysfunction and oxidative stress are commonly found in key tissues (pancreatic islets, liver, and skeletal muscle) of IUGR individuals. In this review, we explore the role of oxidative stress in IUGR-associated diabetes etiology.
Figures
References
-
- Alcántara AI, Morales M, Delgado E, López-Delgado MI, Clemente F, Luque MA, Malaisse WJ, Valverde I, Villanueva-Peñacarrillo ML. Exendin-4 agonist and exendin(9-39)amide antagonist of the GLP-1(7-36)amide effects in liver and muscle. Arch Biochem Biophys 341: 1–7, 1997. doi:10.1006/abbi.1997.9951. - DOI - PubMed
-
- Bansal A, Rashid C, Xin F, Li C, Polyak E, Duemler A, van der Meer T, Stefaniak M, Wajid S, Doliba N, Bartolomei MS, Simmons RA. Sex- and dose-specific effects of maternal bisphenol A exposure on pancreatic islets of first- and second-generation adult mice offspring. Environ Health Perspect 125: 097022, 2017. doi:10.1289/EHP1674. - DOI - PMC - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

