Defining the Rhythmogenic Elements of Mammalian Breathing
- PMID: 30109823
- PMCID: PMC6230551
- DOI: 10.1152/physiol.00025.2018
Defining the Rhythmogenic Elements of Mammalian Breathing
Abstract
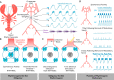
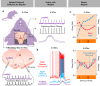
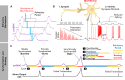
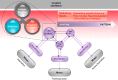
Breathing's remarkable ability to adapt to changes in metabolic, environmental, and behavioral demands stems from a complex integration of its rhythm-generating network within the wider nervous system. Yet, this integration complicates identification of its specific rhythmogenic elements. Based on principles learned from smaller rhythmic networks of invertebrates, we define criteria that identify rhythmogenic elements of the mammalian breathing network and discuss how they interact to produce robust, dynamic breathing.
Figures


References
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

