An Introduction to the Structure and Function of the Catalytic Core Enzyme of Escherichia coli RNA Polymerase
- PMID: 30109846
- PMCID: PMC6095464
- DOI: 10.1128/ecosalplus.ESP-0004-2018
An Introduction to the Structure and Function of the Catalytic Core Enzyme of Escherichia coli RNA Polymerase
Abstract
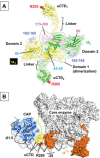
RNA polymerase (RNAP) is the essential enzyme responsible for transcribing genetic information stored in DNA to RNA. Understanding the structure and function of RNAP is important for those who study basic principles in gene expression, such as the mechanism of transcription and its regulation, as well as translational sciences such as antibiotic development. With over a half-century of investigations, there is a wealth of information available on the structure and function of Escherichia coli RNAP. This review introduces the structural features of E. coli RNAP, organized by subunit, giving information on the function, location, and conservation of these features to early stage investigators who have just started their research of E. coli RNAP.
Figures



References
-
- Fujita N, Ishihama A. 1996. Reconstitution of RNA polymerase. Methods Enzymol 273:121–130. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

