Assessment of Right Ventricular Function in the Research Setting: Knowledge Gaps and Pathways Forward. An Official American Thoracic Society Research Statement
- PMID: 30109950
- PMCID: PMC6835085
- DOI: 10.1164/rccm.201806-1160ST
Assessment of Right Ventricular Function in the Research Setting: Knowledge Gaps and Pathways Forward. An Official American Thoracic Society Research Statement
Abstract
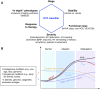
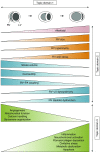
Background: Right ventricular (RV) adaptation to acute and chronic pulmonary hypertensive syndromes is a significant determinant of short- and long-term outcomes. Although remarkable progress has been made in the understanding of RV function and failure since the meeting of the NIH Working Group on Cellular and Molecular Mechanisms of Right Heart Failure in 2005, significant gaps remain at many levels in the understanding of cellular and molecular mechanisms of RV responses to pressure and volume overload, in the validation of diagnostic modalities, and in the development of evidence-based therapies.
Methods: A multidisciplinary working group of 20 international experts from the American Thoracic Society Assemblies on Pulmonary Circulation and Critical Care, as well as external content experts, reviewed the literature, identified important knowledge gaps, and provided recommendations.
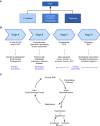
Results: This document reviews the knowledge in the field of RV failure, identifies and prioritizes the most pertinent research gaps, and provides a prioritized pathway for addressing these preclinical and clinical questions. The group identified knowledge gaps and research opportunities in three major topic areas: 1) optimizing the methodology to assess RV function in acute and chronic conditions in preclinical models, human studies, and clinical trials; 2) analyzing advanced RV hemodynamic parameters at rest and in response to exercise; and 3) deciphering the underlying molecular and pathogenic mechanisms of RV function and failure in diverse pulmonary hypertension syndromes.
Conclusions: This statement provides a roadmap to further advance the state of knowledge, with the ultimate goal of developing RV-targeted therapies for patients with RV failure of any etiology.
Keywords: acute respiratory distress syndrome; pulmonary circulation; pulmonary embolism; pulmonary hypertension; right ventricle.
Figures

References
-
- Voelkel NF, Quaife RA, Leinwand LA, Barst RJ, McGoon MD, Meldrum DR, et al. National Heart, Lung, and Blood Institute Working Group on Cellular and Molecular Mechanisms of Right Heart Failure. Right ventricular function and failure: report of a National Heart, Lung, and Blood Institute working group on cellular and molecular mechanisms of right heart failure. Circulation. 2006;114:1883–1891. - PubMed
-
- Vonk-Noordegraaf A, Haddad F, Chin KM, Forfia PR, Kawut SM, Lumens J, et al. Right heart adaptation to pulmonary arterial hypertension: physiology and pathobiology. J Am Coll Cardiol. 2013;62:D22–D33. - PubMed
-
- van de Veerdonk MC, Kind T, Marcus JT, Mauritz GJ, Heymans MW, Bogaard HJ, et al. Progressive right ventricular dysfunction in patients with pulmonary arterial hypertension responding to therapy. J Am Coll Cardiol. 2011;58:2511–2519. - PubMed
-
- Riedel M, Stanek V, Widimsky J, Prerovsky I. Longterm follow-up of patients with pulmonary thromboembolism: late prognosis and evolution of hemodynamic and respiratory data. Chest. 1982;81:151–158. - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

