Chemokine CXCL10 and Coronavirus-Induced Neurologic Disease
- PMID: 30109979
- PMCID: PMC6350065
- DOI: 10.1089/vim.2018.0073
Chemokine CXCL10 and Coronavirus-Induced Neurologic Disease
Abstract
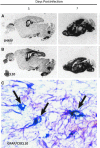
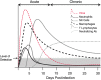
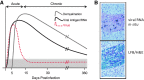
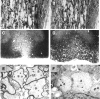
Chemokines (chemotactic cytokines) are involved in a wide variety of biological processes. Following microbial infection, there is often robust chemokine signaling elicited from infected cells, which contributes to both innate and adaptive immune responses that control growth of the invading pathogen. Infection of the central nervous system (CNS) by the neuroadapted John Howard Mueller (JHM) strain of mouse hepatitis virus (JHMV) provides an excellent example of how chemokines aid in host defense as well as contribute to disease. Intracranial inoculation of the CNS of susceptible mice with JHMV results in an acute encephalomyelitis characterized by widespread dissemination of virus throughout the parenchyma. Virus-specific T cells are recruited to the CNS, and control viral replication through release of antiviral cytokines and cytolytic activity. Sterile immunity is not acquired, and virus will persist primarily in white matter tracts leading to chronic neuroinflammation and demyelination. Chemokines are expressed and contribute to defense as well as chronic disease by attracting targeted populations of leukocytes to the CNS. The T cell chemoattractant chemokine CXCL10 (interferon-inducible protein 10 kDa, IP-10) is prominently expressed in both stages of disease, and serves to attract activated T and B lymphocytes expressing CXC chemokine receptor 3 (CXCR3), the receptor for CXCL10. Functional studies that have blocked expression of either CXCL10 or CXCR3 illuminate the important role of this signaling pathway in host defense and neurodegeneration in a model of viral-induced neurologic disease.
Keywords: CNS infection; CXCL10; coronavirus.
Conflict of interest statement
No competing financial interests exist.
Figures



References
-
- Akwa Y, Hassett DE, Eloranta ML, et al. . Transgenic expression of IFN-alpha in the central nervous system of mice protects against lethal neurotropic viral infection but induces inflammation and neurodegeneration. J Immunol 1998;161:5016–5026 - PubMed
-
- Baerwald KD, and Popko B. Developing and mature oligodendrocytes respond differently to the immune cytokine interferon-gamma. J Neurosci Res 1998;52:230–239 - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

