CheckMate-032 Study: Efficacy and Safety of Nivolumab and Nivolumab Plus Ipilimumab in Patients With Metastatic Esophagogastric Cancer
- PMID: 30110194
- PMCID: PMC6161834
- DOI: 10.1200/JCO.2017.76.6212
CheckMate-032 Study: Efficacy and Safety of Nivolumab and Nivolumab Plus Ipilimumab in Patients With Metastatic Esophagogastric Cancer
Erratum in
-
Erratum.J Clin Oncol. 2019 Feb 10;37(5):443. doi: 10.1200/JCO.18.02406. J Clin Oncol. 2019. PMID: 30726682 Free PMC article. No abstract available.
Abstract
Purpose: Metastatic esophagogastric cancer treatments after failure of second-line chemotherapy are limited. Nivolumab demonstrated superior overall survival (OS) versus placebo in Asian patients with advanced gastric or gastroesophageal junction cancers. We assessed the safety and efficacy of nivolumab and nivolumab plus ipilimumab in Western patients with chemotherapy-refractory esophagogastric cancers.
Patients and methods: Patients with locally advanced or metastatic chemotherapy-refractory gastric, esophageal, or gastroesophageal junction cancer from centers in the United States and Europe received nivolumab or nivolumab plus ipilimumab. The primary end point was objective response rate. The association of tumor programmed death-ligand 1 status with response and survival was also evaluated.
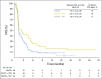
Results: Of 160 treated patients (59 with nivolumab 3 mg/kg, 49 with nivolumab 1 mg/kg plus ipilimumab 3 mg/kg, 52 with nivolumab 3 mg/kg plus ipilimumab 1 mg/kg), 79% had received two or more prior therapies. At the data cutoff, investigator-assessed objective response rates were 12% (95% CI, 5% to 23%), 24% (95% CI, 13% to 39%), and 8% (95% CI, 2% to 19%) in the three groups, respectively. Responses were observed regardless of tumor programmed death-ligand 1 status. With a median follow-up of 28, 24, and 22 months across the three groups, 12-month progression-free survival rates were 8%, 17%, and 10%, respectively; 12-month OS rates were 39%, 35%, and 24%, respectively. Treatment-related grade 3/4 adverse events were reported in 17%, 47%, and 27% of patients in the three groups, respectively.
Conclusion: Nivolumab and nivolumab plus ipilimumab demonstrated clinically meaningful antitumor activity, durable responses, encouraging long-term OS, and a manageable safety profile in patients with chemotherapy-refractory esophagogastric cancer. Phase III studies evaluating nivolumab or nivolumab plus ipilimumab in earlier lines of therapy for esophagogastric cancers are underway.
Trial registration: ClinicalTrials.gov NCT01928394.
Figures






Comment in
-
A balancing act: dual immune-checkpoint inhibition for oesophagogastric cancer.Nat Rev Clin Oncol. 2019 Jan;16(1):9-10. doi: 10.1038/s41571-018-0108-x. Nat Rev Clin Oncol. 2019. PMID: 30291292 No abstract available.
-
Immune checkpoint inhibitors in esophagogastric adenocarcinoma: do the results justify the hype?J Thorac Dis. 2018 Dec;10(12):6407-6411. doi: 10.21037/jtd.2018.12.01. J Thorac Dis. 2018. PMID: 30746176 Free PMC article. No abstract available.
-
Immune checkpoint inhibitors in esophagogastric cancer: still a long way to go.J Thorac Dis. 2019 Feb;11(2):351-353. doi: 10.21037/jtd.2018.11.111. J Thorac Dis. 2019. PMID: 30962971 Free PMC article. No abstract available.
-
Immuno-checkpoint inhibitors in metastatic esophago-gastric cancer.J Thorac Dis. 2019 Mar;11(Suppl 3):S376-S380. doi: 10.21037/jtd.2018.12.71. J Thorac Dis. 2019. PMID: 30997225 Free PMC article. No abstract available.
-
CheckMate-032 Study: promising efficacy with nivolumab-based immunotherapy in pretreated esophagogastric cancer.J Thorac Dis. 2019 Mar;11(Suppl 3):S394-S395. doi: 10.21037/jtd.2018.12.02. J Thorac Dis. 2019. PMID: 30997229 Free PMC article. No abstract available.
References
-
- International Agency for Research on Cancer: Stomach cancer: GLOBOCAN 2012: Estimated incidence, mortality and prevalence worldwide in 2012. http://globocan.iarc.fr/Pages/fact_sheets_cancer.aspx?cancer=stomach.
-
- International Agency for Research on Cancer: Oesophageal cancer: GLOBOCAN 2012: Estimated incidence, mortality and prevalence. http://globocan.iarc.fr/Pages/fact_sheets_cancer.aspx?cancer=oesophagus.
-
- Anandappa G, Chau I: Emerging novel therapeutic agents in the treatment of patients with gastroesophageal and gastric adenocarcinoma. Hematol Oncol Clin North Am 31:529-544, 2017 - PubMed
Publication types
MeSH terms
Substances
Associated data
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials

