Neuronal population mechanisms of lightness perception
- PMID: 30110233
- PMCID: PMC6295546
- DOI: 10.1152/jn.00906.2017
Neuronal population mechanisms of lightness perception
Abstract
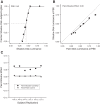
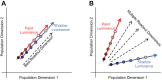
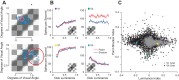
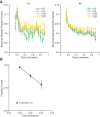
The way that humans and animals perceive the lightness of an object depends on its physical luminance as well as its surrounding context. While neuronal responses throughout the visual pathway are modulated by context, the relationship between neuronal responses and lightness perception is poorly understood. We searched for a neuronal mechanism of lightness by recording responses of neuronal populations in monkey primary visual cortex (V1) and area V4 to stimuli that produce a lightness illusion in humans, in which the lightness of a disk depends on the context in which it is embedded. We found that the way individual units encode the luminance (or equivalently for our stimuli, contrast) of the disk and its context is extremely heterogeneous. This motivated us to ask whether the population representation in either V1 or V4 satisfies three criteria: 1) disk luminance is represented with high fidelity, 2) the context surrounding the disk is also represented, and 3) the representations of disk luminance and context interact to create a representation of lightness that depends on these factors in a manner consistent with human psychophysical judgments of disk lightness. We found that populations of units in both V1 and V4 fulfill the first two criteria but that we cannot conclude that the two types of information in either area interact in a manner that clearly predicts human psychophysical measurements: the interpretation of our population measurements depends on how subsequent areas read out lightness from the population responses. NEW & NOTEWORTHY A core question in visual neuroscience is how the brain extracts stable representations of object properties from the retinal image. We searched for a neuronal mechanism of lightness perception by determining whether the responses of neuronal populations in primary visual cortex and area V4 could account for a lightness illusion measured using human psychophysics. Our results suggest that comparing psychophysics with population recordings will yield insight into neuronal mechanisms underlying a variety of perceptual phenomena.
Keywords: lightness; population coding; psychophysics; visual cortex.
Figures


References
-
- Adelson EH. Lightness perception and lightness illusions. In: The New Cognitive Neurosciences (2nd ed.), edited by Gazzaniga M. Cambridge, MA: MIT Press, 2000, p. 339–351
-
- Brainard DH, Pelli D, Robson T. Display characterization. In Encylopedia of Imaging Science and Technology, edited by Hornak J. New York: Wiley, 2002, p. 172–188. doi:10.1002/0471443395.img011 - DOI
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

