FMRFa receptor stimulated Ca2+ signals alter the activity of flight modulating central dopaminergic neurons in Drosophila melanogaster
- PMID: 30110323
- PMCID: PMC6110513
- DOI: 10.1371/journal.pgen.1007459
FMRFa receptor stimulated Ca2+ signals alter the activity of flight modulating central dopaminergic neurons in Drosophila melanogaster
Abstract
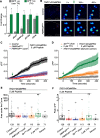
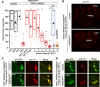
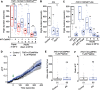
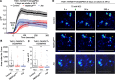
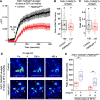
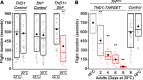
Neuropeptide signaling influences animal behavior by modulating neuronal activity and thus altering circuit dynamics. Insect flight is a key innate behavior that very likely requires robust neuromodulation. Cellular and molecular components that help modulate flight behavior are therefore of interest and require investigation. In a genetic RNAi screen for G-protein coupled receptors that regulate flight bout durations, we earlier identified several receptors, including the receptor for the neuropeptide FMRFa (FMRFaR). To further investigate modulation of insect flight by FMRFa we generated CRISPR-Cas9 mutants in the gene encoding the Drosophila FMRFaR. The mutants exhibit significant flight deficits with a focus in dopaminergic cells. Expression of a receptor specific RNAi in adult central dopaminergic neurons resulted in progressive loss of sustained flight. Further, genetic and cellular assays demonstrated that FMRFaR stimulates intracellular calcium signaling through the IP3R and helps maintain neuronal excitability in a subset of dopaminergic neurons for positive modulation of flight bout durations.
Conflict of interest statement
The authors have declared that no competing interests exist.
Figures

Similar articles
-
Modulation of flight and feeding behaviours requires presynaptic IP3Rs in dopaminergic neurons.Elife. 2020 Nov 6;9:e62297. doi: 10.7554/eLife.62297. Elife. 2020. PMID: 33155978 Free PMC article.
-
A genetic RNAi screen for IP₃/Ca²⁺ coupled GPCRs in Drosophila identifies the PdfR as a regulator of insect flight.PLoS Genet. 2013;9(10):e1003849. doi: 10.1371/journal.pgen.1003849. Epub 2013 Oct 3. PLoS Genet. 2013. PMID: 24098151 Free PMC article.
-
Store-Operated Calcium Entry through Orai Is Required for Transcriptional Maturation of the Flight Circuit in Drosophila.J Neurosci. 2015 Oct 7;35(40):13784-99. doi: 10.1523/JNEUROSCI.1680-15.2015. J Neurosci. 2015. PMID: 26446229 Free PMC article.
-
The genetics of calcium signaling in Drosophila melanogaster.Biochim Biophys Acta. 2012 Aug;1820(8):1269-82. doi: 10.1016/j.bbagen.2011.11.002. Epub 2011 Nov 7. Biochim Biophys Acta. 2012. PMID: 22100727 Review.
-
Identifying neuropeptide and protein hormone receptors in Drosophila melanogaster by exploiting genomic data.Brief Funct Genomic Proteomic. 2006 Feb;4(4):321-30. doi: 10.1093/bfgp/eli003. Epub 2006 Feb 3. Brief Funct Genomic Proteomic. 2006. PMID: 17202123 Review.
Cited by
-
Drosophila neuronal Glucose-6-Phosphatase is a modulator of neuropeptide release that regulates muscle glycogen stores via FMRFamide signaling.Proc Natl Acad Sci U S A. 2024 Jul 23;121(30):e2319958121. doi: 10.1073/pnas.2319958121. Epub 2024 Jul 15. Proc Natl Acad Sci U S A. 2024. PMID: 39008673 Free PMC article.
-
Orai-mediated calcium entry determines activity of central dopaminergic neurons by regulation of gene expression.Elife. 2024 Jan 30;12:RP88808. doi: 10.7554/eLife.88808. Elife. 2024. PMID: 38289659 Free PMC article.
-
Dietary cysteine drives body fat loss via FMRFamide signaling in Drosophila and mouse.Cell Res. 2023 Jun;33(6):434-447. doi: 10.1038/s41422-023-00800-8. Epub 2023 Apr 13. Cell Res. 2023. PMID: 37055592 Free PMC article.
-
Modulation of flight and feeding behaviours requires presynaptic IP3Rs in dopaminergic neurons.Elife. 2020 Nov 6;9:e62297. doi: 10.7554/eLife.62297. Elife. 2020. PMID: 33155978 Free PMC article.
-
Transcriptional profiling of Drosophila male-specific P1 (pC1) neurons.bioRxiv [Preprint]. 2023 Nov 10:2023.11.07.566045. doi: 10.1101/2023.11.07.566045. bioRxiv. 2023. PMID: 37986870 Free PMC article. Preprint.
References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials
Miscellaneous

