Antidepressive and anxiolytic effects of ostruthin, a TREK-1 channel activator
- PMID: 30110354
- PMCID: PMC6093650
- DOI: 10.1371/journal.pone.0201092
Antidepressive and anxiolytic effects of ostruthin, a TREK-1 channel activator
Abstract
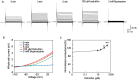
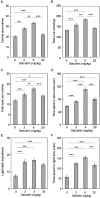
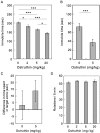
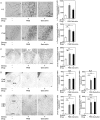
We screened a library of botanical compounds purified from plants of Vietnam for modulators of the activity of a two-pore domain K+ channel, TREK-1, and we identified a hydroxycoumarin-related compound, ostruthin, as an activator of this channel. Ostruthin increased whole-cell TREK-1 channel currents in 293T cells at a low concentration (EC50 = 5.3 μM), and also activity of the TREK-2 channel (EC50 = 3.7 mM). In contrast, ostruthin inhibited other K+ channels, e.g. human ether-à-go-go-related gene (HERG1), inward-rectifier (Kir2.1), voltage-gated (Kv1.4), and two-pore domain (TASK-1) at higher concentrations, without affecting voltage-gated potassium channel (KCNQ1 and 3). We tested the effect of this compound on mouse anxiety- and depression-like behaviors and found anxiolytic activity in the open-field, elevated plus maze, and light/dark box tests. Of note, ostruthin also showed antidepressive effects in the forced swim and tail suspension tests, although previous studies reported that inhibition of TREK-1 channels resulted in an antidepressive effect. The anxiolytic and antidepressive effect was diminished by co-administration of a TREK-1 blocker, amlodipine, indicating the involvement of TREK-1 channels. Administration of ostruthin suppressed the stress-induced increase in anti-c-Fos immunoreactivity in the lateral septum, without affecting immunoreactivity in other mood disorder-related nuclei, e.g. the amygdala, paraventricular nuclei, and dorsal raphe nucleus. Ostruthin may exert its anxiolytic and antidepressive effects through a different mechanism from current drugs.
Conflict of interest statement
The authors have declared that no competing interests exist.
Figures



References
-
- Owen R, Tyrer P. Benzodiazepine dependence. Drugs. 1983;25(4):385–98. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases

