Distance-independent Cross-correlated Relaxation and Isotropic Chemical Shift Modulation in Protein Dynamics Studies
- PMID: 30110510
- PMCID: PMC9206835
- DOI: 10.1002/cphc.201800602
Distance-independent Cross-correlated Relaxation and Isotropic Chemical Shift Modulation in Protein Dynamics Studies
Abstract
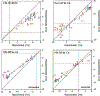
Cross-correlated relaxation (CCR) in multiple-quantum coherences differs from other relaxation phenomena in its theoretical ability to be mediated across an infinite distance. The two interfering relaxation mechanisms may be dipolar interactions, chemical shift anisotropies, chemical shift modulations or quadrupolar interactions. These properties make multiple-quantum CCR an attractive probe for structure and dynamics of biomacromolecules not accessible from other measurements. Here, we review the use of multiple-quantum CCR measurements in dynamics studies of proteins. We compile a list of all experiments proposed for CCR rate measurements, provide an overview of the theory with a focus on protein dynamics, and present applications to various protein systems.
Keywords: biomacromolecules; correlated motion; cross-correlated relaxation; multiple-quantum coherence; protein dynamics.
© 2019 Wiley-VCH Verlag GmbH & Co. KGaA, Weinheim.
Figures




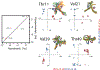





References
-
- Originally coined by Albert Einstein “Spukhafte Fernwirkung” in a letter to Max Born expressing his dissatisfaction with the concept of quantum entanglement.
-
- Stoll ME, Vega AJ, Vaughan RW, J. Chem. Phys 1977, 67, 2029–2038.
-
- Wokaun A, Ernst RR, Mol. Phys 1978, 36, 317–341.
-
- Reif B, Hennig M, Griesinger C, Science 1997, 276, 1230–1233. - PubMed
-
- Schwalbe H, Carlomagno T, Hennig M, Junker J, Reif B, Richter C, Griesinger C, Methods Enzymol 2001, 338, 35–81. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

