Development of a quadruple qRT-PCR assay for simultaneous identification of highly and low pathogenic H7N9 avian influenza viruses and characterization against oseltamivir resistance
- PMID: 30111290
- PMCID: PMC6094886
- DOI: 10.1186/s12879-018-3302-7
Development of a quadruple qRT-PCR assay for simultaneous identification of highly and low pathogenic H7N9 avian influenza viruses and characterization against oseltamivir resistance
Abstract
Background: During the fifth wave of human H7N9 infections, a novel highly pathogenic (HP) H7N9 variant emerged with an insertion of multiple basic amino acids in the HA cleavage site. Moreover, a neuraminidase inhibitor (NAI) resistance (R292K in NA) mutation was found in H7N9 isolates from humans, poultry and the environment. In this study, we set out to develop and validate a multiplex quantitative reverse transcript polymerase chain reaction (qRT-PCR) to simultaneously detect the presence of H7N9 and further identify the HP and NAI-resistance mutations.
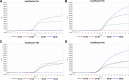
Methods: A quadruple qRT-PCR to simultaneously detect the presence of H7N9 and further identify the HP and NAI-resistance mutations was designed based on the analyses of the HA and NA genes of H7N9. This assay was further tested for specificity and sensitivity, and validated using clinical samples.
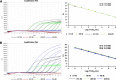
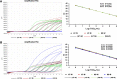
Results: The assay was highly specific and able to detect low pathogenic (LP)- or HP-H7N9 with/without the NAI-resistance mutation. The detection limit of the assay was determined to be 50 genome-equivalent copies and 2.8 × 10- 3 50% tissue culture infectious doses (TCID50) of live H7N9 per reaction. Clinical validation was confirmed by commercial kits and Sanger sequencing with ten clinical samples.
Conclusions: We developed and validated a rapid, single-reaction, one-step, quadruple real-time qRT-PCR to simultaneously detect the presence of H7N9 and further identify the HP- and NAI-resistance strains with excellent performance in specificity and sensitivity. This assay could be used to monitor the evolution of H7N9 viruses in the laboratory, field and the clinic for early-warning and the prevention of H7N9 infections.
Keywords: H7N9; Highly/low pathogenic avian influenza virus; Molecular diagnostics; NAI-resistance; Oseltamivir; Quadruple qRT-PCR.
Conflict of interest statement
Ethics approval and consent to participate
The study was performed in accordance with guidelines approved by the Ethics Committees from Shenzhen Third People’s Hospital (SZTHEC2016001) and Yunnan Center for Disease Control and Prevention Ethics Committee (YNCDC2017001), and verbal informed consents were obtained from all patients or patients’ family members.
Consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing interests.
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Figures
References
-
- Wang X, Jiang H, Wu P, Uyeki TM, Feng L, Lai S, Wang L, Huo X, Xu K, Chen E, et al. Epidemiology of avian influenza a H7N9 virus in human beings across five epidemics in mainland China, 2013-17: an epidemiological study of laboratory-confirmed case series. Lancet Infect Dis. 2017;17(8):822–832. doi: 10.1016/S1473-3099(17)30323-7. - DOI - PMC - PubMed
-
- WHO. Monthly risk assessment summary. 2017. http://www.who.int/influenza/human_animal_interface/HAI_Risk_Assessment/en/. Accessed 4 Nov 2017.
MeSH terms
Substances
Grants and funding
- 2016YFE0205800/Intramural special grant for influenza virus research from the Chinese Academy of Sciences/International
- 2016YFE0205800/National Key Research and Development Project of China/International
- 2016ZX10004222/National Science and Technology Major Project/International
- ZDSYS201504301534057/Sanming Project of Medicine in Shenzhen/International
- JCYJ20160427153238750/Shenzhen Science and Technology Research and Development Project/International
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials
Miscellaneous

