Colorectal liver metastases: surgery versus thermal ablation (COLLISION) - a phase III single-blind prospective randomized controlled trial
- PMID: 30111304
- PMCID: PMC6094448
- DOI: 10.1186/s12885-018-4716-8
Colorectal liver metastases: surgery versus thermal ablation (COLLISION) - a phase III single-blind prospective randomized controlled trial
Abstract
Background: Radiofrequency ablation (RFA) and microwave ablation (MWA) are widely accepted techniques to eliminate small unresectable colorectal liver metastases (CRLM). Although previous studies labelled thermal ablation inferior to surgical resection, the apparent selection bias when comparing patients with unresectable disease to surgical candidates, the superior safety profile, and the competitive overall survival results for the more recent reports mandate the setup of a randomized controlled trial. The objective of the COLLISION trial is to prove non-inferiority of thermal ablation compared to hepatic resection in patients with at least one resectable and ablatable CRLM and no extrahepatic disease.
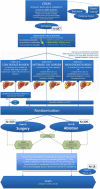
Methods: In this two-arm, single-blind multi-center phase-III clinical trial, six hundred and eighteen patients with at least one CRLM (≤3 cm) will be included to undergo either surgical resection or thermal ablation of appointed target lesion(s) (≤3 cm). Primary endpoint is OS (overall survival, intention-to-treat analysis). Main secondary endpoints are overall disease-free survival (DFS), time to progression (TTP), time to local progression (TTLP), primary and assisted technique efficacy (PTE, ATE), procedural morbidity and mortality, length of hospital stay, assessment of pain and quality of life (QoL), cost-effectiveness ratio (ICER) and quality-adjusted life years (QALY).
Discussion: If thermal ablation proves to be non-inferior in treating lesions ≤3 cm, a switch in treatment-method may lead to a reduction of the post-procedural morbidity and mortality, length of hospital stay and incremental costs without compromising oncological outcome for patients with CRLM.
Trial registration: NCT03088150 , January 11th 2017.
Keywords: Colorectal cancer; Colorectal liver metastases (CRLM); Hepatic resection; Liver metastases; Liver surgery; Microwave ablation (MWA); Radiofrequency ablation (RFA); Thermal ablation.
Conflict of interest statement
Ethics approval and consent to participate
Ethics approval was obtained from the Medisch Ethische Toetsingscommissie (METc) Amsterdam UMC (location VUmc). Reference number 2016.561. NL-number NL58551.029.16. All participants will provide written informed consent.
Consent for publication
Not applicable.
Competing interests
Investigator Sponsored Research (ISR) grant by Medtronic PLC. The funders had no role in the design of the study; the collection, analysis, or interpretation of the data; the writing of the manuscript; or the decision to submit the manuscript for publication.
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
-
- Comprehensive Cancer Organisation the Netherlands (I.K.N.L.). National evidence-based guideline. Colorectaalcarcinoom. Available from: https://www.oncoline.nl/colorectaalcarcinoom.
-
- Belinson S, Chopra R, Yang Y, Shankaran V, Aronson N. Local Hepatic Therapies for Metastases to the Liver From Unresectable Colorectal Cancer. AHRQ Comparative Effectiveness Reviews. Rockville: Agency for Healthcare Research and Quality (US); 2012. Report No.: 13-EHC014-EF. - PubMed
Publication types
MeSH terms
Associated data
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials