Exosomes derived from mesenchymal stem cells enhance radiotherapy-induced cell death in tumor and metastatic tumor foci
- PMID: 30111323
- PMCID: PMC6094906
- DOI: 10.1186/s12943-018-0867-0
Exosomes derived from mesenchymal stem cells enhance radiotherapy-induced cell death in tumor and metastatic tumor foci
Abstract
Background: We have recently shown that radiotherapy may not only be a successful local and regional treatment but, when combined with MSCs, may also be a novel systemic cancer therapy. This study aimed to investigate the role of exosomes derived from irradiated MSCs in the delay of tumor growth and metastasis after treatment with MSC + radiotherapy (RT).
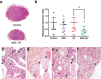
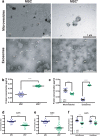
Methods: We have measured tumor growth and metastasis formation, of subcutaneous human melanoma A375 xenografts on NOD/SCID-gamma mice, and the response of tumors to treatment with radiotherapy (2 Gy), mesenchymal cells (MSC), mesenchymal cells plus radiotherapy, and without any treatment. Using proteomic analysis, we studied the cargo of the exosomes released by the MSC treated with 2 Gy, compared with the cargo of exosomes released by MSC without treatment.
Results: The tumor cell loss rates found after treatment with the combination of MSC and RT and for exclusive RT, were: 44.4% % and 12,1%, respectively. Concomitant and adjuvant use of RT and MSC, increased the mice surviving time 22,5% in this group, with regard to the group of mice treated with exclusive RT and in a 45,3% respect control group. Moreover, the number of metastatic foci found in the internal organs of the mice treated with MSC + RT was 60% less than the mice group treated with RT alone. We reasoned that the exosome secreted by the MSC, could be implicated in tumor growth delay and metastasis control after treatment.
Conclusions: Our results show that exosomes derived form MSCs, combined with radiotherapy, are determinant in the enhancement of radiation effects observed in the control of metastatic spread of melanoma cells and suggest that exosome-derived factors could be involved in the bystander, and abscopal effects found after treatment of the tumors with RT plus MSC. Radiotherapy itself may not be systemic, although it might contribute to a systemic effect when used in combination with mesenchymal stem cells owing the ability of irradiated MSCs-derived exosomes to increase the control of tumor growth and metastasis.
Keywords: Abscopal effect; Annexin A1; Bystander effect; Cell therapy; Experimental radiotherapy; Melanoma xenograft; Mesenchymal stem cells; Metastasis spread; Proteomic analysis.
Conflict of interest statement
Ethics approval and consent to participate
These studies were performed in strict accordance with the recommendations of the Guide for the Care and Use of Laboratory Animals of the Bioethical Committee of Granada University, and the protocol was approved by the Committee on the Ethics of Animal Experiments of the CSIC.
Consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing interests.
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Figures





References
-
- Burnet NG, Wurm R, Nyman J, Peacock JH: Normal tissue radiosensitivity--how important is it? Clinical oncology (Royal College of Radiologists (Great Britain)) 1996, 8:25–34. - PubMed
-
- Lopez E, Guerrero R, Nunez MI, del Moral R, Villalobos M, Martinez-Galan J, Valenzuela MT, Munoz-Gamez JA, Oliver FJ, Martin-Oliva D, Ruiz de Almodovar JM. early and late skin reactions to radiotherapy for breast cancer and their correlation with radiation-induced DNA damage in lymphocytes. Breast Cancer Res. 2005;7:R690–R698. doi: 10.1186/bcr1277. - DOI - PMC - PubMed
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

