Human lung development: recent progress and new challenges
- PMID: 30111617
- PMCID: PMC6124546
- DOI: 10.1242/dev.163485
Human lung development: recent progress and new challenges
Abstract
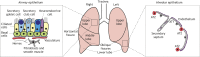
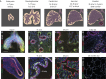
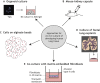
Recent studies have revealed biologically significant differences between human and mouse lung development, and have reported new in vitro systems that allow experimental manipulation of human lung models. At the same time, emerging clinical data suggest that the origins of some adult lung diseases are found in embryonic development and childhood. The convergence of these research themes has fuelled a resurgence of interest in human lung developmental biology. In this Review, we discuss our current understanding of human lung development, which has been profoundly influenced by studies in mice and, more recently, by experiments using in vitro human lung developmental models and RNA sequencing of human foetal lung tissue. Together, these approaches are helping to shed light on the mechanisms underlying human lung development and disease, and may help pave the way for new therapies.
Keywords: Alveolar; Bronchi; ESC; Lung disease; Progenitor; Stem cell; iPSC.
© 2018. Published by The Company of Biologists Ltd.
Conflict of interest statement
Competing interestsThe authors declare no competing or financial interests.
Figures


References
-
- Ardini-Poleske M. E., Clark R. F., Ansong C., Carson J. P., Corley R. A., Deutsch G. H., Hagood J. S., Kaminski N., Mariani T. J., Potter S. S. et al. (2017). LungMAP: the molecular atlas of lung development program. Am. J. Physiol. Lung Cell Mol. Physiol. 313, L733-L740. 10.1152/ajplung.00139.2017 - DOI - PMC - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

