Staphylococcus aureus Biofilm Growth on Cystic Fibrosis Airway Epithelial Cells Is Enhanced during Respiratory Syncytial Virus Coinfection
- PMID: 30111629
- PMCID: PMC6094059
- DOI: 10.1128/mSphere.00341-18
Staphylococcus aureus Biofilm Growth on Cystic Fibrosis Airway Epithelial Cells Is Enhanced during Respiratory Syncytial Virus Coinfection
Abstract
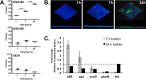
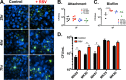
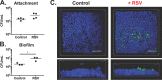
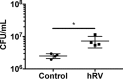
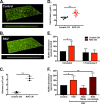
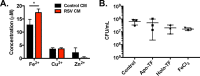
Staphylococcus aureus is a major cause of chronic respiratory infection in patients with cystic fibrosis (CF). We recently showed that Pseudomonas aeruginosa exhibits enhanced biofilm formation during respiratory syncytial virus (RSV) coinfection on human CF airway epithelial cells (AECs). The impact of respiratory viruses on other bacterial pathogens during polymicrobial infections in CF remains largely unknown. To investigate if S. aureus biofilm growth in the CF airways is impacted by virus coinfection, we evaluated S. aureus growth on CF AECs. Initial studies showed an increase in S. aureus growth over 24 h, and microscopy revealed biofilm-like clusters of bacteria on CF AECs. Biofilm growth was enhanced when CF AECs were coinfected with RSV, and this observation was confirmed with S. aureus CF clinical isolates. Apical conditioned medium from RSV-infected cells promoted S. aureus biofilms in the absence of the host epithelium, suggesting that a secreted factor produced during virus infection benefits S. aureus biofilms. Exogenous iron addition did not significantly alter biofilm formation, suggesting that it is not likely the secreted factor. We further characterized S. aureus-RSV coinfection in our model using dual host-pathogen RNA sequencing, allowing us to observe specific contributions of S. aureus and RSV to the host response during coinfection. Using the dual host-pathogen RNA sequencing approach, we observed increased availability of nutrients from the host and upregulation of S. aureus genes involved in growth, protein translation and export, and amino acid metabolism during RSV coinfection.IMPORTANCE The airways of individuals with cystic fibrosis (CF) are commonly chronically infected, and Staphylococcus aureus is the dominant bacterial respiratory pathogen in CF children. CF patients also experience frequent respiratory virus infections, and it has been hypothesized that virus coinfection increases the severity of S. aureus lung infections in CF. We investigated the relationship between S. aureus and the CF airway epithelium and observed that coinfection with respiratory syncytial virus (RSV) enhances S. aureus biofilm growth. However, iron, which was previously found to be a significant factor influencing Pseudomonas aeruginosa biofilms during virus coinfection, plays a minor role in S. aureus coinfections. Transcriptomic analyses provided new insight into how bacterial and viral pathogens alter host defense and suggest potential pathways by which dampening of host responses to one pathogen may favor persistence of another in the CF airways, highlighting complex interactions occurring between bacteria, viruses, and the host during polymicrobial infections.
Keywords: biofilms; coinfection; host-pathogen interaction; polymicrobial.
Copyright © 2018 Kiedrowski et al.
Figures


Similar articles
-
Pseudomonas aeruginosa Alginate Overproduction Promotes Coexistence with Staphylococcus aureus in a Model of Cystic Fibrosis Respiratory Infection.mBio. 2017 Mar 21;8(2):e00186-17. doi: 10.1128/mBio.00186-17. mBio. 2017. PMID: 28325763 Free PMC article.
-
Association of Diverse Staphylococcus aureus Populations with Pseudomonas aeruginosa Coinfection and Inflammation in Cystic Fibrosis Airway Infection.mSphere. 2021 Jun 30;6(3):e0035821. doi: 10.1128/mSphere.00358-21. Epub 2021 Jun 23. mSphere. 2021. PMID: 34160233 Free PMC article.
-
Respiratory syncytial virus infection enhances Pseudomonas aeruginosa biofilm growth through dysregulation of nutritional immunity.Proc Natl Acad Sci U S A. 2016 Feb 9;113(6):1642-7. doi: 10.1073/pnas.1516979113. Epub 2016 Jan 4. Proc Natl Acad Sci U S A. 2016. PMID: 26729873 Free PMC article.
-
Viral-Bacterial Co-infections in the Cystic Fibrosis Respiratory Tract.Front Immunol. 2018 Dec 20;9:3067. doi: 10.3389/fimmu.2018.03067. eCollection 2018. Front Immunol. 2018. PMID: 30619379 Free PMC article. Review.
-
The role of Staphylococcus aureus in cystic fibrosis pathogenesis and clinico-microbiological interactions.Diagn Microbiol Infect Dis. 2024 Jul;109(3):116294. doi: 10.1016/j.diagmicrobio.2024.116294. Epub 2024 Apr 4. Diagn Microbiol Infect Dis. 2024. PMID: 38678689 Review.
Cited by
-
Phage Therapy for Multi-Drug Resistant Respiratory Tract Infections.Viruses. 2021 Sep 11;13(9):1809. doi: 10.3390/v13091809. Viruses. 2021. PMID: 34578390 Free PMC article. Review.
-
Genetic requirements and transcriptomics of Helicobacter pylori biofilm formation on abiotic and biotic surfaces.NPJ Biofilms Microbiomes. 2020 Nov 27;6(1):56. doi: 10.1038/s41522-020-00167-3. NPJ Biofilms Microbiomes. 2020. PMID: 33247117 Free PMC article.
-
Elevated glucose increases methicillin-resistant Staphylococcus aureus antibiotic tolerance in a cystic fibrosis airway epithelial cell infection model.Res Sq [Preprint]. 2025 Feb 17:rs.3.rs-5938603. doi: 10.21203/rs.3.rs-5938603/v1. Res Sq. 2025. PMID: 40034435 Free PMC article. Preprint.
-
SARS-CoV-2 Infection in Patients with Cystic Fibrosis: What We Know So Far.Life (Basel). 2022 Dec 13;12(12):2087. doi: 10.3390/life12122087. Life (Basel). 2022. PMID: 36556452 Free PMC article. Review.
-
RSV enhances Staphylococcus aureus bacterial growth in the lung.Infect Immun. 2024 Oct 15;92(10):e0030424. doi: 10.1128/iai.00304-24. Epub 2024 Aug 16. Infect Immun. 2024. PMID: 39150268 Free PMC article.
References
-
- Cystic Fibrosis Foundation 2016. 2015 CFF patient registry annual data report. Cystic Fibrosis Foundation, Bethesda, MD.
-
- Chmiel JF, Aksamit TR, Chotirmall SH, Dasenbrook EC, Elborn JS, LiPuma JJ, Ranganathan SC, Waters VJ, Ratjen FA. 2014. Antibiotic management of lung infections in cystic fibrosis. I. The microbiome, methicillin-resistant Staphylococcus aureus, Gram-negative bacteria, and multiple infections. Ann Am Thorac Soc 11:1120–1129. doi:10.1513/AnnalsATS.201402-050AS. - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

