A protective Langerhans cell-keratinocyte axis that is dysfunctional in photosensitivity
- PMID: 30111646
- PMCID: PMC6365282
- DOI: 10.1126/scitranslmed.aap9527
A protective Langerhans cell-keratinocyte axis that is dysfunctional in photosensitivity
Abstract
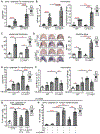
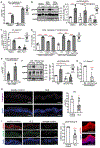
Photosensitivity, or skin sensitivity to ultraviolet radiation (UVR), is a feature of lupus erythematosus and other autoimmune and dermatologic conditions, but the mechanistic underpinnings are poorly understood. We identify a Langerhans cell (LC)-keratinocyte axis that limits UVR-induced keratinocyte apoptosis and skin injury via keratinocyte epidermal growth factor receptor (EGFR) stimulation. We show that the absence of LCs in Langerin-diphtheria toxin subunit A (DTA) mice leads to photosensitivity and that, in vitro, mouse and human LCs can directly protect keratinocytes from UVR-induced apoptosis. LCs express EGFR ligands and a disintegrin and metalloprotease 17 (ADAM17), the metalloprotease that activates EGFR ligands. Deletion of ADAM17 from LCs leads to photosensitivity, and UVR induces LC ADAM17 activation and generation of soluble active EGFR ligands, suggesting that LCs protect by providing activated EGFR ligands to keratinocytes. Photosensitive systemic lupus erythematosus (SLE) models and human SLE skin show reduced epidermal EGFR phosphorylation and LC defects, and a topical EGFR ligand reduces photosensitivity. Together, our data establish a direct tissue-protective function for LCs, reveal a mechanistic basis for photosensitivity, and suggest EGFR stimulation as a treatment for photosensitivity in lupus erythematosus and potentially other autoimmune and dermatologic conditions.
Copyright © 2018 The Authors, some rights reserved; exclusive licensee American Association for the Advancement of Science. No claim to original U.S. Government Works.
Figures

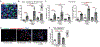



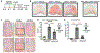
References
-
- Chong BF, Werth VP, in Dubois' Lupus Erythematosus and Related Syndromes (Eighth Edition), Hahn BH, Ed. (W.B. Saunders, Philadelphia, 2013), pp. 319–332.
-
- Millard TP, Hawk JLM, Photosensitivity disorders: cause, effect, and management. Am J Clin Dermatol 3, 239–246 (2002). - PubMed
-
- Kuhn A, Wenzel J, Weyd H, Photosensitivity, Apoptosis, and Cytokines in the Pathogenesis of Lupus Erythematosus: a Critical Review. Clin Rev Allerg Immu, 1–15 (2014). - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials
Miscellaneous

