Modulating plant growth-metabolism coordination for sustainable agriculture
- PMID: 30111841
- PMCID: PMC6155485
- DOI: 10.1038/s41586-018-0415-5
Modulating plant growth-metabolism coordination for sustainable agriculture
Abstract
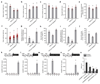
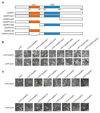
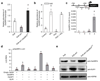
Enhancing global food security by increasing the productivity of green revolution varieties of cereals risks increasing the collateral environmental damage produced by inorganic nitrogen fertilizers. Improvements in the efficiency of nitrogen use of crops are therefore essential; however, they require an in-depth understanding of the co-regulatory mechanisms that integrate growth, nitrogen assimilation and carbon fixation. Here we show that the balanced opposing activities and physical interactions of the rice GROWTH-REGULATING FACTOR 4 (GRF4) transcription factor and the growth inhibitor DELLA confer homeostatic co-regulation of growth and the metabolism of carbon and nitrogen. GRF4 promotes and integrates nitrogen assimilation, carbon fixation and growth, whereas DELLA inhibits these processes. As a consequence, the accumulation of DELLA that is characteristic of green revolution varieties confers not only yield-enhancing dwarfism, but also reduces the efficiency of nitrogen use. However, the nitrogen-use efficiency of green revolution varieties and grain yield are increased by tipping the GRF4-DELLA balance towards increased GRF4 abundance. Modulation of plant growth and metabolic co-regulation thus enables novel breeding strategies for future sustainable food security and a new green revolution.
Conflict of interest statement
Figures












Comment in
-
Improved nutrient use gives cereal crops a boost.Nature. 2018 Aug;560(7720):563-564. doi: 10.1038/d41586-018-05928-x. Nature. 2018. PMID: 30143754 No abstract available.
References
-
- Khush GS. Green revolution: preparing for the 21st century. Genome. 1999;42:646–655. - PubMed
-
- Evenson RE, Gollin D. Assessing the impact of the green revolution, 1960 to 2000. Science. 2003;300:758–762. - PubMed
-
- Hedden P. The genes of the Green Revolution. Trends Genet. 2003;19:5–9. - PubMed
-
- Peng J, et al. Green revolution genes encode mutant gibberellin response modulators. Nature. 1999;400:256–261. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials

