Clinical and Molecular Characteristics Associated with Survival in Advanced Melanoma Treated with Checkpoint Inhibitors
- PMID: 30112001
- PMCID: PMC6077519
- DOI: 10.1155/2018/6279871
Clinical and Molecular Characteristics Associated with Survival in Advanced Melanoma Treated with Checkpoint Inhibitors
Abstract
Background: We performed meta-analysis to gather more evidence regarding clinical-molecular subgroups associated with better overall survival (OS) in advanced melanoma treated with checkpoint inhibitors.
Materials and methods: We performed a systematic search of PubMed, Scopus, Cochrane Library, and clinical trial.gov. Randomized clinical trials that compared a checkpoint inhibitor (nivolumab or pembrolizumab) with investigator choice chemotherapy or ipilimumab were included in our study. Hazard ratios (HR) and confidence interval (CI) were calculated for progression-free survival (PFS) and OS for each subgroup using generic inverse model along with the random effect method.
Results: A total of 6 clinical trials were eligible for the meta-analysis. OS was prolonged in wild BRAF subgroup (HR 0.65, 95% CI 0.49-0.85, p 0.002), Programmed cell death subgroup (PD-1+) (HR 0.57, 95% CI 0.41-0.80, p 0.001), and high lactate dehydrogenase (LDH) level subgroup (HR 0.60, 95% CI 0.38-0.95, p 0.03). Similarly, we found increased OS in eastern cooperative oncology group (ECOG) 1, males and age >65 years subgroups.
Conclusions: Checkpoint inhibitors significantly increased OS in patients with wild BRAF, positive PD-1, and high LDH. However, results should be interpreted keeping in mind associated significant heterogeneity. The results of this study should help in designing future clinical trials.
Figures
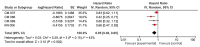
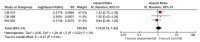
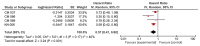



References
-
- Howlader N., Noone Am., Krapcho M., et al. SEER Data Submission, Posted to the SEER Web Site. SEER Cancer Statistics Review. April 2017:1975–2014. http://seer.cancer.gov/csr/1975_2014/
-
- Hauschild A., Jean-Jacques G., Lev Demidov V., et al. Dabrafenib in BRAF-Mutated Metastatic Melanoma: A Multicentre, Open-Label, Phase 3 Randomised Controlled Trial. The Lancet. 2018:358–365. - PubMed
Publication types
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials

