Exendin-4 protects INS-1 cells against palmitate-induced apoptosis through the IRE1α-Xbp1 signaling pathway
- PMID: 30112049
- PMCID: PMC6090425
- DOI: 10.3892/etm.2018.6240
Exendin-4 protects INS-1 cells against palmitate-induced apoptosis through the IRE1α-Xbp1 signaling pathway
Abstract
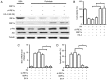
The anti-apoptotic effect of the incretin analog, exendin-4 (EX-4) on pancreatic β cells is mediated via the activation of protein kinase B (Akt) signaling, and its effect is partly produced through the inhibition of endoplasmic reticulum (ER) stress. However, the molecular mechanisms that underlie the effect of EX-4 on the suppression of ER stress and the upregulation of Akt signaling are poorly understood. Inositol-requiring enzyme 1 (IRE1), a member of the ER-localized transmembrane protein family, activates its downstream transcription factor X-box binding protein 1 (XBP1) to mediate a key part of the cellular unfolded protein response in order to cope with ER stress. Using the clonal rat pancreatic β cell line INS-1, the present study produced an in vitro model of ER stress using palmitate (PA) in order to determine whether the beneficial effect of EX-4 under ER stress was regulated by the IRE1α-Xbp1 signaling pathway. The results demonstrated that the reduction in ER stress and the activation Akt by EX-4 may be associated with the upregulation of IRE1α phosphorylation and the splicing of Xbp1 mRNA, which improved PA-reduced cell viability. This effect was partially abrogated by the knockdown of IRE1α with small interfering RNA. Additionally, cellular IRE1α was phosphorylated by the protein kinase A (PKA) associated with EX-4 and the activation of IRE1α, as IRE1α phosphorylation was attenuated by the inhibition of PKA with its inhibitor. In conclusion, the data identified the IRE1α-Xbp1 signaling pathway as an essential mediator that associates EX-4 with the intracellular mechanism that inhibits ER stress and activates Akt in order to regulate β cell survival. This may provide important evidence for the use of EX-4 in treatments for type 2 diabetes.
Keywords: X-box binding protein 1; apoptosis; endoplasmic reticulum stress; exendine-4; inositol-requiring enzyme 1α.
Figures



References
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
