Recent advances in hypervalent iodine(III)-catalyzed functionalization of alkenes
- PMID: 30112085
- PMCID: PMC6071704
- DOI: 10.3762/bjoc.14.154
Recent advances in hypervalent iodine(III)-catalyzed functionalization of alkenes
Abstract
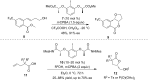
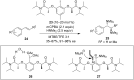
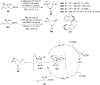
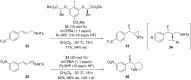
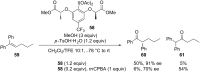
Hypervalent iodine(III) reagents have been well-developed and widely utilized in functionalization of alkenes, however, generally either stoichiometric amounts of iodine(III) reagents are required or stoichiometric oxidants such as mCPBA are employed to in situ generate iodine(III) species. In this review, recent developments of hypervalent iodine(III)-catalyzed functionalization of alkenes and asymmetric reactions using a chiral iodoarene are summarized.
Keywords: asymmetric catalysis; functionalization of alkenes; hypervalent iodine(III).
Figures


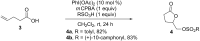



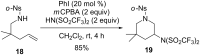






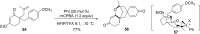


Similar articles
-
Hypervalent iodine-catalyzed oxidative functionalizations including stereoselective reactions.Chem Asian J. 2014 Apr;9(4):950-71. doi: 10.1002/asia.201301582. Epub 2014 Feb 12. Chem Asian J. 2014. PMID: 24523252
-
Hypervalent Iodine Reagents in Palladium-Catalyzed Oxidative Cross-Coupling Reactions.Front Chem. 2020 Sep 29;8:705. doi: 10.3389/fchem.2020.00705. eCollection 2020. Front Chem. 2020. PMID: 33134246 Free PMC article. Review.
-
Palladium-Catalyzed Organic Reactions Involving Hypervalent Iodine Reagents.Molecules. 2022 Jun 17;27(12):3900. doi: 10.3390/molecules27123900. Molecules. 2022. PMID: 35745020 Free PMC article. Review.
-
Alkene Difunctionalization Using Hypervalent Iodine Reagents: Progress and Developments in the Past Ten Years.Molecules. 2019 Jul 19;24(14):2634. doi: 10.3390/molecules24142634. Molecules. 2019. PMID: 31331092 Free PMC article. Review.
-
Iodanyl Radical Catalysis.Acc Chem Res. 2023 Jul 18;56(14):2026-2036. doi: 10.1021/acs.accounts.3c00231. Epub 2023 Jul 6. Acc Chem Res. 2023. PMID: 37409761 Free PMC article.
Cited by
-
Halogen Complexes of Anionic N-Heterocyclic Carbenes.Chemistry. 2021 Mar 1;27(13):4349-4363. doi: 10.1002/chem.202004418. Epub 2020 Dec 21. Chemistry. 2021. PMID: 33094865 Free PMC article.
-
Enantio- and Regioselective Palladium(II)-Catalyzed Dioxygenation of (Aza-)Alkenols.Angew Chem Int Ed Engl. 2021 Sep 27;60(40):21723-21727. doi: 10.1002/anie.202109312. Epub 2021 Sep 6. Angew Chem Int Ed Engl. 2021. PMID: 34387928 Free PMC article.
-
Mechanism and Origins of Chemo- and Stereoselectivities of Aryl Iodide-Catalyzed Asymmetric Difluorinations of β-Substituted Styrenes.J Am Chem Soc. 2018 Nov 14;140(45):15206-15218. doi: 10.1021/jacs.8b05935. Epub 2018 Nov 5. J Am Chem Soc. 2018. PMID: 30350956 Free PMC article.
-
Recent discoveries on the structure of iodine(iii) reagents and their use in cross-nucleophile coupling.Chem Sci. 2021 Jan 7;12(3):853-864. doi: 10.1039/d0sc03266b. Chem Sci. 2021. PMID: 34163852 Free PMC article. Review.
-
Predicting bond dissociation energies of cyclic hypervalent halogen reagents using DFT calculations and graph attention network model.Beilstein J Org Chem. 2024 Jun 28;20:1444-1452. doi: 10.3762/bjoc.20.127. eCollection 2024. Beilstein J Org Chem. 2024. PMID: 38952960 Free PMC article.
References
-
- Varvoglis A. The Organic Chemistry of Polycoordinated Iodine. New York: VCH Publishers, Inc.; 1992.
-
- Akiba K-y., editor. Chemistry of Hypervalent Compounds. New York: Wiley-VCH; 1999.
-
- Zhdankin V V, Stang P J. In: Chemistry of Hypervalent Compounds. Akiba K-y., editor. New York: VCH Publishers; 1999.
-
- Ochiai M. In: Chemistry of Hypervalent Compounds. Akiba K-y., editor. New York: VCH Publishers; 1999.
-
- Varvoglis A. Tetrahedron. 2010;66:5739–5744. doi: 10.1016/j.tet.2010.04.126. - DOI
Publication types
LinkOut - more resources
Full Text Sources
Other Literature Sources
