Biophysical, Biochemical, and Cell Based Approaches Used to Decipher the Role of Carbonic Anhydrases in Cancer and to Evaluate the Potency of Targeted Inhibitors
- PMID: 30112206
- PMCID: PMC6077552
- DOI: 10.1155/2018/2906519
Biophysical, Biochemical, and Cell Based Approaches Used to Decipher the Role of Carbonic Anhydrases in Cancer and to Evaluate the Potency of Targeted Inhibitors
Abstract
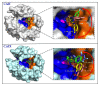
Carbonic anhydrases (CAs) are thought to be important for regulating pH in the tumor microenvironment. A few of the CA isoforms are upregulated in cancer cells, with only limited expression in normal cells. For these reasons, there is interest in developing inhibitors that target these tumor-associated CA isoforms, with increased efficacy but limited nonspecific cytotoxicity. Here we present some of the biophysical, biochemical, and cell based techniques and approaches that can be used to evaluate the potency of CA targeted inhibitors and decipher the role of CAs in tumorigenesis, cancer progression, and metastatic processes. These techniques include esterase activity assays, stop flow kinetics, and mass inlet mass spectroscopy (MIMS), all of which measure enzymatic activity of purified protein, in the presence or absence of inhibitors. Also discussed is the application of X-ray crystallography and Cryo-EM as well as other structure-based techniques and thermal shift assays to the studies of CA structure and function. Further, large-scale genomic and proteomic analytical methods, as well as cell based techniques like those that measure cell growth, apoptosis, clonogenicity, and cell migration and invasion, are discussed. We conclude by reviewing approaches that test the metastatic potential of CAs and how the aforementioned techniques have contributed to the field of CA cancer research.
Figures



Similar articles
-
Design of two-tail compounds with rotationally fixed benzenesulfonamide ring as inhibitors of carbonic anhydrases.Eur J Med Chem. 2018 Aug 5;156:61-78. doi: 10.1016/j.ejmech.2018.06.059. Epub 2018 Jun 27. Eur J Med Chem. 2018. PMID: 30006175
-
Thermodynamic, kinetic, and structural parameterization of human carbonic anhydrase interactions toward enhanced inhibitor design.Q Rev Biophys. 2018 Jan;51:e10. doi: 10.1017/S0033583518000082. Q Rev Biophys. 2018. PMID: 30912486 Review.
-
Functionalization of fluorinated benzenesulfonamides and their inhibitory properties toward carbonic anhydrases.ChemMedChem. 2015 Apr;10(4):662-87. doi: 10.1002/cmdc.201402490. Epub 2015 Mar 10. ChemMedChem. 2015. PMID: 25758852
-
Carbonic anhydrases: current state of the art, therapeutic applications and future prospects.J Enzyme Inhib Med Chem. 2004 Jun;19(3):199-229. doi: 10.1080/14756360410001689540. J Enzyme Inhib Med Chem. 2004. PMID: 15499993 Review.
-
Structural study of interaction between brinzolamide and dorzolamide inhibition of human carbonic anhydrases.Bioorg Med Chem. 2013 Nov 15;21(22):7210-5. doi: 10.1016/j.bmc.2013.08.033. Epub 2013 Aug 28. Bioorg Med Chem. 2013. PMID: 24090602
Cited by
-
Targeting the pH Paradigm at the Bedside: A Practical Approach.Int J Mol Sci. 2020 Dec 3;21(23):9221. doi: 10.3390/ijms21239221. Int J Mol Sci. 2020. PMID: 33287221 Free PMC article. Review.
-
Picomolar fluorescent probes for compound affinity determination to carbonic anhydrase IX expressed in live cancer cells.Sci Rep. 2022 Oct 21;12(1):17644. doi: 10.1038/s41598-022-22436-1. Sci Rep. 2022. PMID: 36271018 Free PMC article.
-
Role of Carbonic Anhydrases and Inhibitors in Acid-Base Physiology: Insights from Mathematical Modeling.Int J Mol Sci. 2019 Aug 6;20(15):3841. doi: 10.3390/ijms20153841. Int J Mol Sci. 2019. PMID: 31390837 Free PMC article. Review.
References
Publication types
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

